Revolutionary Breakthrough: Scientists Capture a Single Electron’s Movement during a Chemical Reaction!
2025-08-29
Author: Arjun
A Historic First in Electron Imaging
In a groundbreaking achievement, scientists have successfully captured the movement of a single electron during a chemical reaction, utilizing ultrafast X-ray flashes. This remarkable research, published on August 20 in Physical Review Letters, marks a monumental leap in our understanding of atomic-scale reactions.
Unlocking the Secrets of Chemically Active Electrons
The team, led by physics doctoral student Ian Gabalski, focused on a valence electron—the outermost electron responsible for chemical reactions. For years, scientists have struggled to visualize these elusive particles, as traditional X-ray techniques predominantly interacted with core electrons located near an atom's nucleus.
Why Ammonia? The Perfect Candidate for Imaging!
For their stunning experiment, the researchers chose ammonia as their target molecule. Gabalski explained, "Ammonia is special because it contains mainly light atoms, allowing us to isolate the signal from the valence electrons without interference from core electrons." This strategic choice was pivotal for their imaging success.
The Experiment: Ultraviolet Light and X-ray Precision
Conducted at the SLAC National Accelerator Laboratory's Linac Coherent Light Source, the experiment began with a pulse of ultraviolet light that excited an electron to a higher energy state, instigating a chemical reaction. Following this, the researchers unleashed intense X-ray pulses to monitor the rapid changes in the electron’s ‘cloud’ as the ammonia molecule disintegrated.
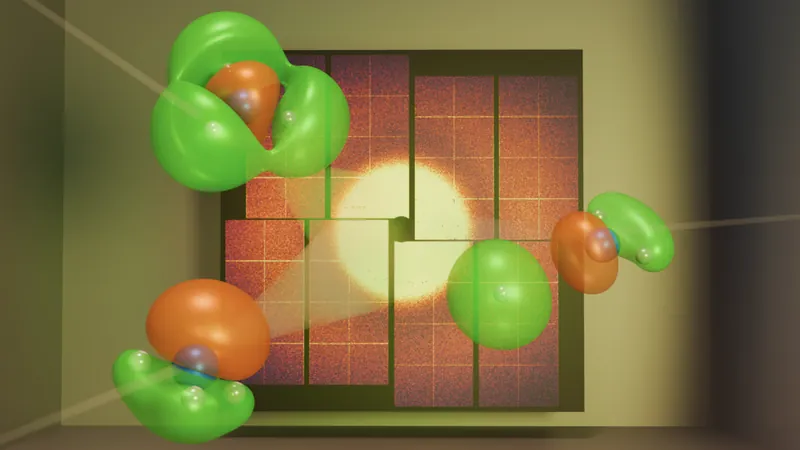
Mapping the Electron's Probability Cloud
In the quantum realm, electrons are not mere tiny spheres but exist as probability clouds with varying densities, indicating where they are likely to be found. To visualize this cloud, Gabalski and his team utilized quantum mechanical simulations to model the molecule's electronic structure, allowing them to determine how the electron filled its surrounding orbitals.
Reconstructing Electron Movement Using X-ray Interference
As X-rays passed through the electron's probability cloud, they scattered in various directions. By analyzing the interference patterns created by these scattering events, the researchers were able to reconstruct images of the electron's orbital and observe its real-time movement during the reaction.
A Game-Changer for Drug Design and Regenerative Medicine!
The implications of this discovery are monumental. By understanding valence electron movements, scientists could pave the way for designing advanced pharmaceuticals, developing cleaner chemical processes, and creating more efficient materials. Furthermore, they aim to adapt this groundbreaking technique for use in more complex, three-dimensional environments to enhance applications in regenerative medicine, such as constructing or repairing tissues on demand.
The Future is Bright!
This revolutionary technique not only opens up new avenues in chemistry and material science but also heralds the potential for transformative advances in medicine. The journey to uncover the mysteries of the atomic world is just beginning!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)