Revolutionary Breakthrough: Researchers Achieve Atomic Precision in Bimetallic Catalysts!
2025-01-08
Author: Mei
Revolutionary Breakthrough in Bimetallic Catalysts
In an exciting development in chemistry, researchers have made significant strides in enhancing the performance of bimetallic catalysts, which are crucial for selective heterogeneous hydrogenations. Composed of a noble metal and a base metal, these catalysts possess unique properties due to their distinct geometric and electronic structures. At the heart of efficient and selective hydrogenation is the need for site-specific interactions, where active atoms on the catalyst efficiently engage with targeted functional groups in a substrate.
Innovative Approach
The innovation lies in reducing catalyst particles to nanoscale atomic clusters and crafting single-atom alloys. This miniaturization not only improves surface dispersion but also dramatically enhances the utilization of precious noble metal atoms. Moreover, it transforms the electronic structure of active sites, thereby influencing the catalyst's overall effectiveness and altering product distributions.
Challenges and Solutions
A major challenge in this field has been the precise fabrication of atomic structures that serve as active sites. In a groundbreaking study published in Chem, a team led by Professors Shen Wenjie and Li Yong from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, in collaboration with colleagues from the University of Science and Technology of China and Karlsruhe Institute of Technology in Germany, has tackled this issue head-on.
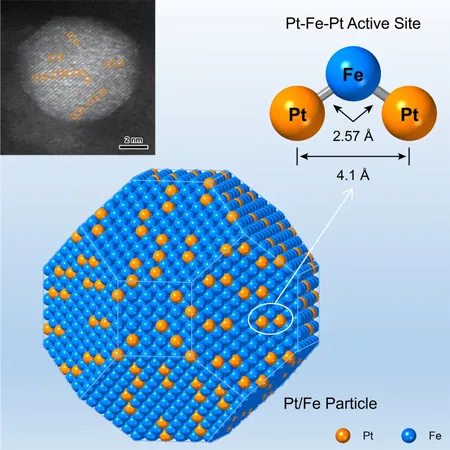
Development of Pt-Fe-Pt Heterotrimers
The researchers developed an innovative method to densely populate and accurately position isolated platinum (Pt) atoms, forming a unique Pt-Fe-Pt heterotrimer on α-Fe nanoparticles. This was achieved through H2-reduction of a composite pair made of Pt and Fe2O3, where a 3.3 nm Pt particle is strategically placed on a 9.8 nm Fe2O3 particle. The reduction process converts iron oxides to elemental iron, which allows the Pt atoms to amalgamate with the iron, evolving into Pt-Fe-Pt heterotrimers on the iron particle surface via surface alloying.
Coordinated Behavior During Hydrogenation
Pooling their insights, the researchers highlighted the coordinated behavior of the Pt-Fe-Pt heterotrimer during gas-phase hydrogenation of crotonaldehyde. Notably, this complex exhibited a strong preference for hydrogenating the carbonyl (C=O) bond, yielding crotyl alcohol over the conjugated C=C bond, resulting in an impressive 35-fold increase in the intrinsic hydrogenation rate—effectively addressing the long-standing activity-selectivity dilemma in hydrogenation reactions.
Site-Bond Recognition Patterns
A fascinating revelation came from the study's exploration of the site-bond recognition pattern within the heterotrimer. The configuration revealed that the Pt atom on the left anchored the C=C bond, while the central Fe atom played a critical role in activating the C=O bond, which was subsequently hydrogenated by protons contributed from the right-end Pt atom.
Implications and Future Directions
"Our study quantifies the surface catalytic reaction at the molecular level, offering a viable strategy for the fine-tuning of active sites on bimetallic catalysts with atomic precision," stated Professor Shen, underscoring the study's potential impactful implications in catalysis and material science.
This research not only marks a pivotal moment in catalysis but also sets the stage for advancements in sustainable chemistry and green technologies. Are we on the verge of a new era in hydrogenation processes? Stay tuned!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)