Revolutionary Breakthrough in Mechanochemical Synthesis: New Theory Unlocks Secrets of Reaction Rate Boosting!
2025-01-24
Author: Daniel
Introduction
In a stunning advancement for the field of organic synthesis, researchers are shedding light on mechanochemistry, a promising method that eliminates the environmental burden of solvents traditionally used in chemical reactions.
Unlike standard organic synthesis, mechanochemistry operates without solvents, significantly reducing industrial waste while allowing the use of solids that are poorly soluble in conventional solvents. This means a greener and more efficient approach to chemical reactions is on the horizon.
Lagging Theories
Until now, the foundational theories behind mechanochemical reactions have lagged behind those of traditional organic synthesis.
While previous studies indicated that the mechanical forces applied in these reactions could accelerate chemical interactions, the specifics of how macroscopic forces influence molecular behavior remained unclear.
Groundbreaking Theory
However, a groundbreaking new theory developed by a team led by Associate Professor Tetsuya Yamamoto from the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) at Hokkaido University may change this narrative.
In their published study in the journal RSC Mechanochemistry, Yamamoto and his collaborators—comprising experts in organic chemistry and rheology—propose a novel theory that predicts the rates of reactions in mechanochemical processes using a ball mill.
Their findings could potentially shift mechanochemistry from a niche technique to a standard practice in organic synthesis, given a more solid theoretical grounding.
Key Insights from the New Theory
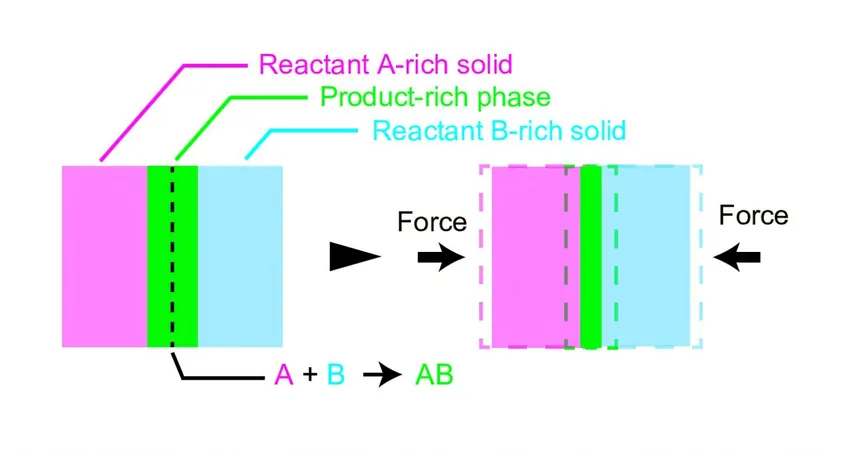
According to this innovative theory, the key to understanding reaction acceleration lies at the interface between solid reactants.
This interface is where the magic happens—chemical reactions occur to produce the final product.
The new model suggests that the rapid collisions caused by the balls in the mill compress the product-rich layer at this interface, effectively reducing its thickness and promoting faster interactions among reactants.
The result? A notable increase in the rate of product formation.
Expert Opinions
"This research marks a pioneering step in establishing a kinetic theory for mechanochemical reactions by focusing on the critical interfaces between reactants," said Associate Professor Tetsuya Yamamoto.
"This foundational understanding holds the potential to propel mechanochemistry into a more recognized strategy for organic synthesis."
Further insights from the study's second author, Associate Professor Koji Kubota, highlight the complexity surrounding mechanochemical processes.
"Unraveling the intricate mechanisms of these reactions has been challenging," he stated.
"Our collaboration at WPI-ICReDD has enabled us to create a preliminary theoretical framework that elucidates the significant role mechanical forces play in accelerating these vital chemical reactions."
Implications and Future Directions
As the scientific community continues to explore the implications of this groundbreaking research, the potential applications for mechanochemical synthesis are vast—from pharmaceuticals to materials science.
The promise of a more sustainable and efficient route in organic synthesis may be closer than ever.
In a world grappling with environmental challenges, this advancement could signify a turning point in how we approach chemistry, offering both economic and ecological benefits.
Conclusion
Stay tuned as we follow the developments in mechanochemistry—this revolutionary field could dramatically reshape how we perceive and execute chemical synthesis!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)