Revolutionary Breakthrough: Cancer Cells 'Hacked' to Trigger Immune Warfare!
2025-03-31
Author: Sarah
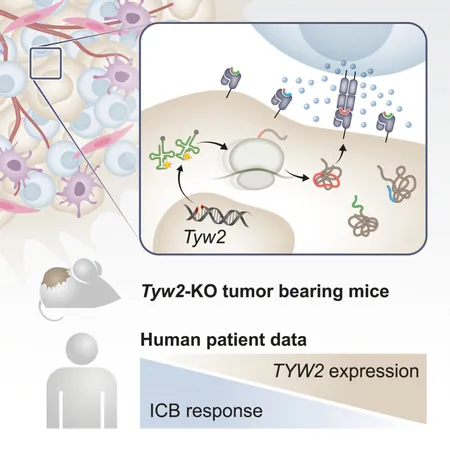
In a groundbreaking advance in cancer treatment, researchers at the Weizmann Institute of Science have discovered a novel method to 'hack' cancer cells, effectively forcing them to reveal their hidden vulnerabilities to the immune system. Just like a social media account can be compromised, cancer cells can also be manipulated to present unusual protein markers that alert the body’s immune defenses. This innovative approach significantly enhances the immune response, potentially transforming the future of cancer therapy.
Cancer cells are notoriously savvy at evading detection. Unlike regular cells that broadcast “tweets” of their health, these rogue cells often parade very few suspicious signals, making it difficult for the immune system to identify and eliminate them. Fortunately, a research team led by Prof. Yardena Samuels has unveiled a technique that bolsters the immune system’s arsenal by deliberately disrupting the protein production process in tumor cells.
Their recent study published in the journal Cancer Cell introduces a method that compels cancer cells to produce a variety of unusual proteins. This disruption not only exposes these malignant cells but also initiates a vigorous immune response capable of battling aggressive tumors, as demonstrated in mice.
Immunotherapy has already been a game-changer in the treatment landscape; however, it remains effective for only a minority of patients. Killer T cells are the foot soldiers of our immune system, patrolling for foreign entities. For them to recognize and attack cancer cells, those mutations must present themselves as clear signals. Unfortunately, many cancers exhibit limited mutations, leaving few targets for T cells to engage.
Prof. Samuels elucidated, "The bizarre proteins derived from disrupted translation may not always root from DNA mutations. We explored intentionally impeding the translation process to amplify the immune system's targets." This translation disruption involves the ribosome, the cellular machine that assembles proteins by interpreting genetic recipes, and it turns out that slight alterations in this process can produce 34 unique proteins specifically generated in cancer cells.
In an animal model, the research team observed that upon disrupting the translation process, an influx of killer T cells swarmed the tumors, indicating a robust immune response. However, a familiar hurdle arose: the T cells grew "exhausted" before conquering the tumor, a challenge that plagues immunotherapy.
To bolster the immune assault, the researchers combined their novel approach with existing immunotherapy treatments. This powerful partnership showed remarkable efficacy, with around 40% of treated mice experiencing tumor eradication. This combination therapy showcases the potential to revamp current treatment regimens, especially for melanoma, which previously resisted available immunotherapeutics.
But the implications extend beyond immediate treatment. Oncologists often categorize patients based on mutation levels, sidelining those with fewer mutations from immunotherapy options. This new discovery indicates that low levels of the enzyme responsible for accurate protein translation could serve as a predictive measure for immunotherapy success. Identifying this could open doors for many patients who previously had limited options.
As the researchers continue their efforts, they are utilizing advanced AI tools in collaboration with Stanford University to discover even more targets for disrupting the translation process. Remarkably, this approach could yield treatments effective across multiple cancer types, from breast and pancreatic cancers to colorectal cancer, as the fundamental translation mechanisms are conserved across different cellular environments.
With fewer than 57% of cancer patients being candidates for current immunotherapy, which is effective in only about 20% of cases, this new strategy is a beacon of hope. The potential to disrupt cancer cells on a molecular level may hold the key to unlocking more effective therapies and ultimately, saving more lives in the ongoing battle against cancer.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)