Revolutionary Breakthrough: Caltech Scientists Transform CO2 Into Plastics with Enhanced Efficiency!
2025-07-16
Author: Jia
For years, the dream of transforming carbon dioxide (CO2) from the atmosphere into valuable products like plastics has captivated scientists and environmentalists alike. While this concept is straightforward in theory, making it work efficiently has been a significant hurdle—until now!
Recent research from Caltech has taken a giant leap forward, significantly improving the efficiency of this process through a groundbreaking two-step method that combines electrocatalysis and thermocatalysis. This innovative approach boasts a CO2 utilization rate of 14%, relying on pure CO2 to drive the transformation.
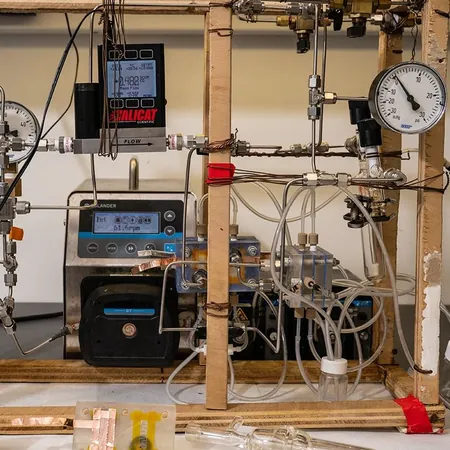
Details spill from the team’s findings published in the prestigious Angewandte Chemie International, though the full study is tucked behind a paywall. However, insightful information can still be gathered from the Supplemental Information. The centerpiece of their research is a remarkable gas diffusion cell (GDE), where copper and silver electrodes interact with CO2 in a potassium bicarbonate (KHCO3) aqueous solution. This reaction leads to the creation of carbon monoxide (CO) and ethylene (C2H4)—two essential building blocks for plastic.
In the subsequent stage, these compounds are processed with a palladium catalyst to produce polyketones, a type of thermoplastic commonly manufactured on an industrial level. What sets this research apart is that ethylene and CO are generated directly in the GDEs, requiring only CO2 and potassium bicarbonate. By recirculating the CO2 for about an hour, the researchers achieve a substantial buildup of these critical gases.
Despite this promising advancement, the researchers caution that the final quality of the polyketones produced still leaves much to be desired. However, this breakthrough could pave the way for more efficient methods to convert greenhouse gases into useful materials, igniting hope for a greener future.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)