Revolutionary Brain Peptide: The Key to Curbing Hunger and Enhancing Glucose Control!
2025-07-26
Author: Li
A Breakthrough Discovery in Hunger Management
In an exciting revelation, researchers from the University of Pennsylvania and Syracuse University have pinpointed a powerful peptide known as octadecaneuropeptide (ODN) that not only curbs appetite but also significantly improves glucose regulation. This newfound peptide, which originates from the hindbrain, promises to tackle obesity and type 2 diabetes without the common side effects of nausea or vomiting.
Transforming Energy Regulation
For ages, the role of ODN in energy balance has been under wraps. However, recent studies illuminate that activating ODN causes monumental weight loss, enhances glucose disposal, and decreases insulin resistance in obese test subjects. Unlike conventional medications that target GLP-1 receptors and bring about unpleasant nausea, ODN works smoothly, making it a game-changer in treating these conditions.
The Struggles of Obesity and Diabetes
Obesity and type 2 diabetes pose sufficient challenges due to their underlying biological mechanisms that sustain high fat mass and blood glucose levels. When individuals attempt weight loss, counter-responses activate increased hunger and a drop in energy expenditure. This is further complicated by sustained high hepatic glucose output even in the face of elevated blood sugar.
The Key Role of Central Energy Sensing
Central to understanding these diseases is how the brain detects nutrient changes. Specific areas, like the hypothalamus and the dorsal vagal complex (DVC), play critical roles in responding to nutrient signals from the gut. In optimal health, these regions support adaptable energy intake and glucose regulation. However, during obesity and diabetes, this process falters, leading to a disruption in homeostasis.
Glial Cells Take Center Stage
Recent research shifts focus toward glial cells, particularly astrocytes and tanycytes, as essential players in nutritional sensing and energy balance. Astrocytes respond to drops in glucose and help suppress feeding, while tanycytes play a role in hormone access, including insulin. Both types produce ODN, which dynamically reacts to glucose levels.
The Research Breakthrough
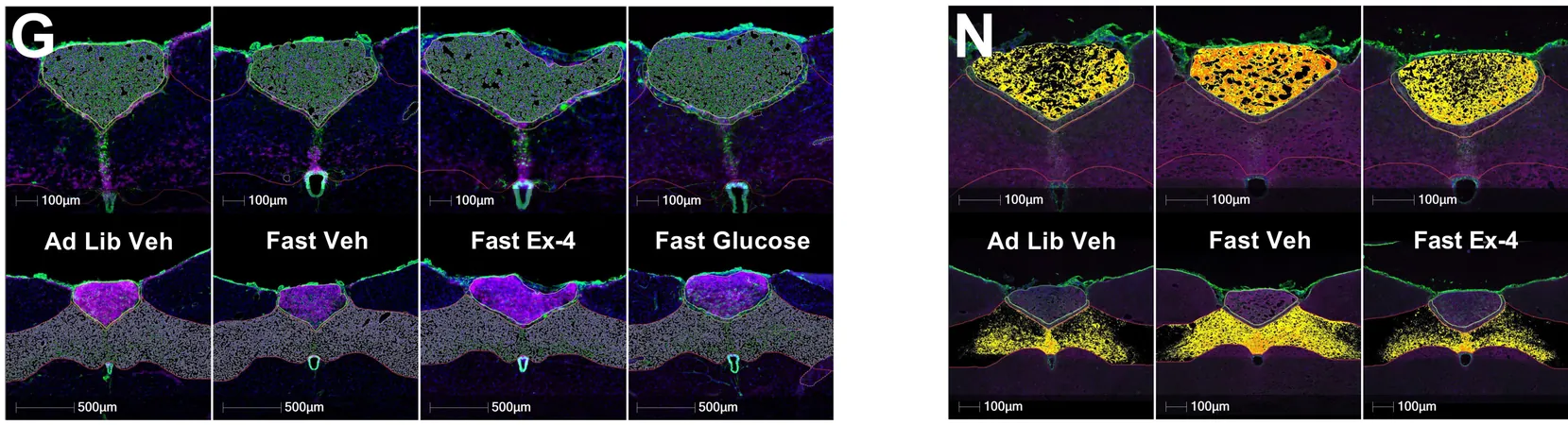
A pivotal study titled 'Hindbrain octadecaneuropeptide gliotransmission as a therapeutic target for energy balance control without nausea or emesis', published in Science Translational Medicine, evaluated whether ODN signaling in the hindbrain could influence feeding behavior, glycemic responses, and hormone levels associated with glucose availability.
Comprehensive Experiments Yield Promising Results
Through a series of experiments involving rats, mice, and musk shrews, researchers injected ODN and its modified form, tridecaneuropeptide (TDN), directly into the brain. Results were astounding: ODN significantly reduced food intake across various diets, improved glucose tolerance, and boosted insulin sensitivity without increasing insulin levels.
Unveiling Mechanisms of Action
The study also highlighted that blocking ODN signaling reversed its beneficial effects, indicating its crucial role in appetite suppression without triggering unpleasant side effects like nausea or increased heart rates. Moreover, TDN also demonstrated the capability to decrease food intake and support weight loss in obese mice.
A New Frontier in Obesity and Diabetes Treatment
The conclusion is clear: targeting the ODN receptors could lead to innovative strategies for combating obesity and type 2 diabetes without the gastrointestinal issues common with existing treatment options. This research not only reveals a promising pathway for future therapies but also potentially marks a turning point in our approach to managing hunger and insulin levels.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)