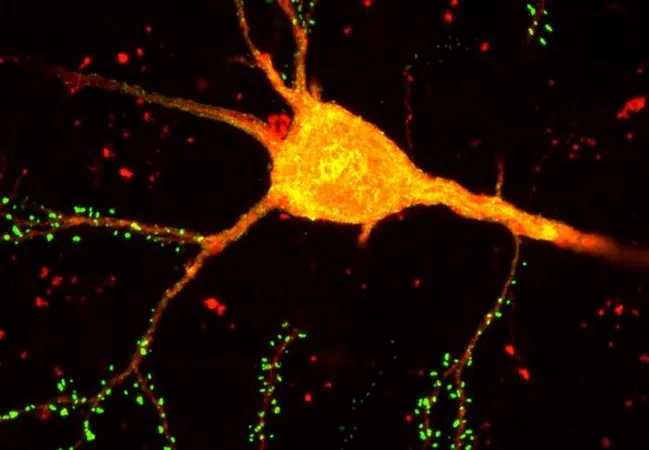
Revolutionary Brain-Mapping Technology START: A Game Changer for Understanding Neuronal Connectivity!
2024-10-01
Overview of START Technology
A groundbreaking neurotechnology, known as Single Transcriptome Assisted Rabies Tracing (START), has emerged as a revolutionary tool in brain mapping, merging monosynaptic rabies virus tracing with single-cell transcriptomics to achieve unprecedented levels of precision in mapping neuronal connections.
Development and Significance
Developed by a team of researchers at the Salk Institute, this cutting-edge method has successfully identified various transcriptomic cell types within the brain, unveiling that local cortical circuit connectivity is intricately tied to specific subclasses and subtypes. This marks a significant advancement, as it is the first time researchers have precisely mapped the patterns of connectivity formed by different transcriptomic subtypes of inhibitory neurons in the cerebral cortex.
Implications for Treatment
But why does this matter? The ability to target neuronal subtypes with such specificity holds the promise of leading to novel therapeutic approaches. Imagine treatments that not only prove to be more effective but also come with far fewer side effects compared to the broader pharmacological methods currently in use.
Publication and Research Results
The breakthrough study, published in the journal Neuron, titled “Transcriptomic Cell-Type Specificity of Local Cortical Circuits,” provides a detailed elucidation of cortical connectivity like never before.
Understanding Neuronal Connectivity
Neuronal interactions rely fundamentally on both excitatory and inhibitory neurons forming networks. While some basic connectivity rules between major neuronal subclasses have been previously identified, the specific connections at the transcriptomic subtype level have remained largely elusive—until now.
Insights from Researchers
Edward Callaway, PhD, a key figure behind this research at Salk, stated, “When it comes to treating neurological and neuropsychiatric disorders, we’ve essentially been trying to fix a machine without fully understanding its parts. START is helping us create a detailed blueprint of the brain’s many parts and how they all connect.”
Fabrication and Methodology
To fabricate the START technology, the research team ingeniously combined methodologies of monosynaptic rabies tracing and single-nuclei RNA sequencing. This approach allows a specially modified virus to traverse from one targeted cell type to its directly connected counterparts.
Applications of START Technology
In their initial applications, researchers explored connectivity within the mouse visual cortex. Astonishingly, START was capable of delineating around 50 distinct inhibitory neuron subtypes in that area and meticulously mapping their connections to excitatory neurons across cortical layers. This achievement unveiled specialized connectivity patterns among various transcriptomic subtypes of inhibitory neurons—insight that previous methods could not provide.
Insights on Inhibitory Neuron Diversity
Maribel Patiño, PhD, a former graduate student in Callaway's lab, noted, "People often treat all inhibitory neurons as a single uniform group, but they’re actually very diverse. Failing to recognize these differences can obscure critical nuances important for brain function and disease."
Unique Connections Found
Furthermore, START illuminated that every layer of excitatory cortical neurons received tailored input from specific inhibitory cells categorized into Sst, Pvalb, Vip, and Lamp5 subtypes. These unique connections likely contribute to refined microcircuits that are pivotal for specialized brain functions.
Notable Discoveries in Sleep Regulation
One remarkable discovery included the Sst Chodl inhibitory subtype, believed to be linked to sleep regulation. The findings revealed that Chodl cells were intricately connected to layer 6 excitatory neurons, which are responsible for thalamic projections essential for sleep rhythm coordination.
Future Directions
So, what's next for the researchers? Their future plans involve creating viral vectors and gene-editing technologies that can specifically target individual cell subtypes. In the foreseeable future, these innovations could evolve into pioneering therapeutics aimed at modifying specific neuronal populations afflicted by disorders like autism, Rett syndrome, and schizophrenia.
Looking Ahead
As Callaway mused, "We don’t know exactly how this information is going to be utilized 10 or 20 years from now, but we know that technological evolution is swift. The methodologies for treating the brain today, primarily through drugs, won't resemble the future approaches. START has the potential to lead that transformation, and we are committed to sharing our resources with the entire neuroscience community."
Conclusion
With the advent of such exciting advancements, the hope for better and more targeted treatments in neurology is shining brighter than ever. Stay tuned as we continue to explore the incredible world of brain science!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)