Revolutionary Blood Test for Early Detection of Parkinson's Disease
2025-04-18
Author: John Tan
A Game-Changer for Neurodegenerative Disease Diagnosis
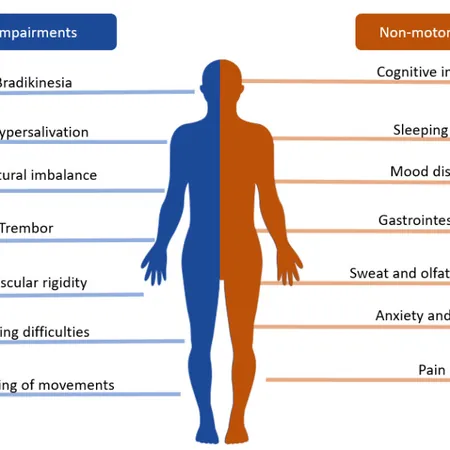
Imagine a future where Parkinson's disease is detected before any symptoms emerge. A groundbreaking study from the Hebrew University of Jerusalem has developed a simple and cost-effective blood test that does just that, potentially transforming how we manage this formidable neurodegenerative condition.
The Problem with Current Diagnoses
Typically, neurodegenerative diseases are diagnosed only after significant neuron loss, making timely treatment nearly impossible—echoing the early days of cancer therapy in the 1970s. This late-stage diagnosis means many patients miss opportunities for early intervention that could significantly improve their quality of life.
How the New Test Works
This innovative blood test detects specific RNA fragments linked to Parkinson's disease. It identifies repetitive RNA sequences that accumulate in patients and measures the decline in mitochondrial RNA levels—a sign of disease progression. By comparing these two biomarkers, the test promises high accuracy, being non-invasive, rapid, and affordable.
Key Players Behind the Breakthrough
The research, spearheaded by doctoral student Nimrod Madrer under the guidance of Professor Hermona Soreq at the prestigious Edmond and Lily Safra Center for Brain Sciences, collaborates with experts from the University of Surrey and Imperial College, London. Together, they have leapfrogged previous methodologies by focusing on transfer RNA fragments (tRFs), which were previously overlooked in Parkinson’s research.
Unveiling New Molecular Insights
The team identified two pivotal biomarkers: an increase in a specific Parkinson's-related tRF and a decrease in mitochondrial tRFs. The test achieved an impressive diagnostic accuracy rate of 0.86 in trials, far surpassing current clinical diagnostic tools, showcasing its potential to distinguish between pre-symptomatic patients and control groups.
Professor Soreq's Insight
Professor Soreq exclaimed, "This discovery represents a major advance in our understanding of Parkinson's disease." By honing in on tRFs, the research illuminates the molecular changes occurring in the earliest stages of the disease.
A Cost-Effective Solution for Healthcare
Utilizing a dual quantitative polymerase chain reaction assay, this test not only prioritizes affordability but also expands access across healthcare facilities. The study revealed that levels of RGTTCRA-tRFs decline following deep brain stimulation, indicating their relevance in both disease mechanisms and treatment responses.
Hope for Patients and Clinicians
Madrer emphasized, "This test has the potential to alleviate the uncertainty faced by patients and clinicians, providing a reliable and rapid method to identify the disease in its earliest stages." Early detection could mean the difference between quality of life and irreversible brain damage.
Future Prospects
The findings have led to the filing of US Provisional Patent Applications, signaling the potential for large-scale clinical validation. With further development, this revolutionary test could soon become a standard of care, ushering in a new era of proactive Parkinson's disease management.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)