Revolutionary Biomarker Transforms Treatment Choices for Multiple Sclerosis Patients
2025-08-11
Author: Wei
Unlocking the Secret to Effective MS Treatment
For those newly diagnosed with multiple sclerosis (MS), a critical decision awaits: should they opt for interferon or glatiramer acetate (GA)? Historically, this choice has felt random—both treatments are well-established, have low side effects, and are generally well-tolerated. Yet, every treatment doesn’t work equally for everyone. But groundbreaking research from the University of Münster has unleashed a powerful tool to guide this decision.
A Genetic Game-Changer
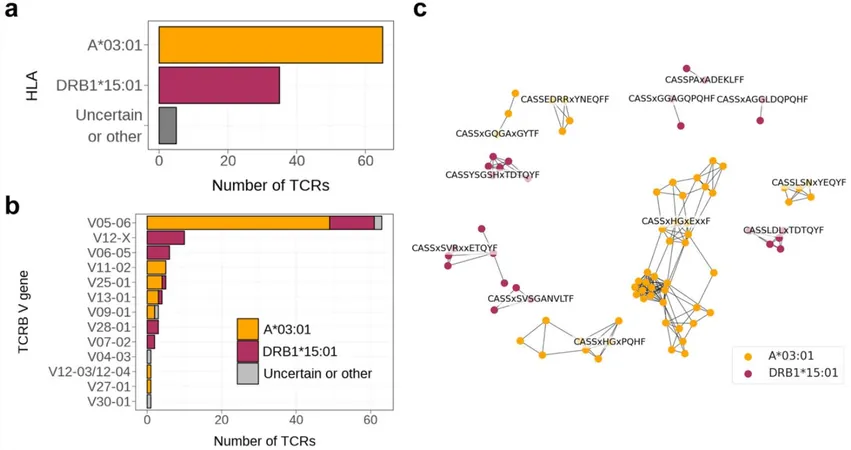
An international team of researchers has pinpointed a genetic biomarker that significantly influences treatment success with glatiramer acetate. Patients possessing the HLA-A*03:01 tissue type show remarkable improvement when treated with GA, outperforming those who receive interferon beta (IFN-β). This pivotal study, examining over 3,000 MS patients, has been unveiled in the esteemed journal eBioMedicine.
Professor Nicholas Schwab, the study leader, remarked, "For the first time, we've connected a genetic marker to the success of an MS treatment, allowing us to predict the optimal medication before therapy even begins." This is a game-changer in the realm of personalized medicine.
The Impact of GA: Confirmed by Rigorous Research
In their detailed analysis, researchers scrutinized T-cell responses in 3,021 MS patients from multiple international cohorts. They discovered that specific T-cell clones emerged exclusively in patients responding positively to GA therapy, linked to the presence of the HLA-A*03:01 or HLA-DRB1*15:01 molecules.
However, only those with the HLA-A*03:01 variant displayed substantial clinical benefits, meaning they genuinely improved following GA treatment.
Significant Findings Across Multiple Cohorts
To ensure their results were robust and applicable across different populations, the team analyzed five extensive cohorts from the U.S., France, and Germany, including the NationMS cohort. The findings were striking: patients carrying the HLA-A*03:01 gene variant reported significantly fewer MS symptoms while on GA compared to IFN-β.
Remarkably, about 30-35% of European MS patients possess this beneficial allele, positioning them to make more informed and effective treatment decisions.
Fast-Track Genetic Testing for Tailored Treatments
What makes this discovery particularly exciting is its immediate clinical applicability. The existing HLA testing, already used for transplant compatibility and drug safety, can conveniently identify the relevant genetic variant.
Furthermore, this study not only introduces a clinically relevant biomarker but also sheds light on the action mechanisms of GA, suggesting that only certain fragments of the drug may be essential for its efficacy. This insight could pave the way for the future development of more targeted and effective therapies in treating MS.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)