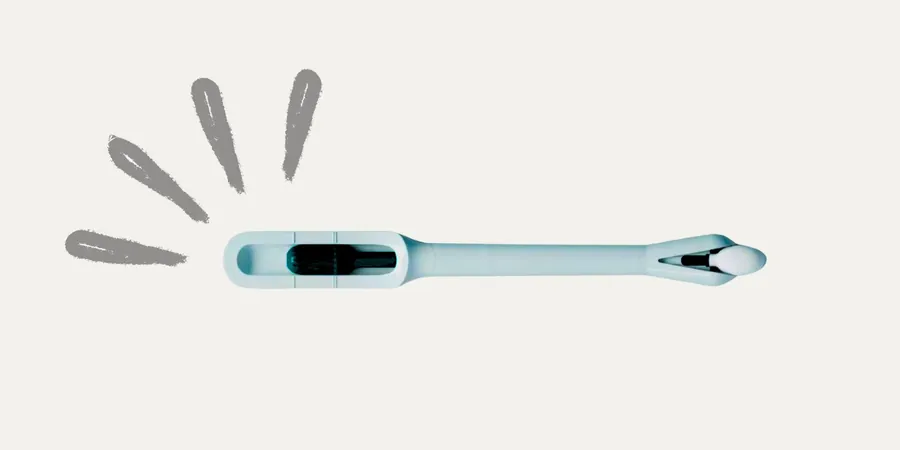
Revolutionary At-Home Device Set to Transform Cervical Cancer Screening
2025-06-03
Author: Daniel
A Game-Changer in Cancer Detection
An innovative at-home self-collection device is stepping up to close the cervical cancer screening gap, and the latest research from the University of Colorado School of Medicine confirms it's not only safe but also strikingly effective.
Dr. Christine Conageski, the leading figure behind this groundbreaking study, emphasizes the urgency of the situation: "Half of new cervical cancer diagnoses arise from women who haven't been screened in the last five years. This highlights a crucial issue—cervical cancer has become a disease of access. We need better access to both screening and vaccines for prevention."
FDA Approval and Trial Success
This remarkable device, known as the Teal Wand, received FDA approval in early May, following a comprehensive trial across 16 medical sites nationwide. Results showed that samples collected at home matched the efficacy of those collected by clinicians, detecting pre-cancerous conditions 96% of the time, and providing a more user-friendly experience than traditional methods.
Addressing the Screening Crisis
Shockingly, an estimated 25% of women are not current on their cervical cancer screenings, often referred to as pap smears. The U.S. Preventive Services Task Force advocates for regular screening every three years for women aged 21-29 and every five years for women aged 30-65.
Dr. Conageski notes the pressing challenge: "We've seen cervical cancer rates stagnate for nearly five years. The screenings simply aren't reaching those at the greatest risk." Cervical cancer is often linked to specific types of human papillomavirus (HPV), transmitted sexually. While many are infected, only a fraction develop cervical cancer—making screenings essential for early detection and intervention.
Unlocking Accessibility
The SELF-CERV trial revealed that 86% of over 600 participants are more likely to keep up with their cervical screenings if they can do them at home. Over 90% preferred the self-collection approach provided by the Teal Wand.
Barriers to in-clinic testing can be daunting, from logistical challenges to emotional discomfort. However, the Teal Wand has demonstrated an ability to alleviate many of these concerns. Participants received a mail kit, allowing them to comfortably collect samples in the privacy of their home and send them back to a laboratory for analysis.
A Rapid and Reliable Solution
Surveys indicated that a third of participants delayed screenings due to scheduling conflicts, discomfort, or financial factors. The Teal Wand has shown to require less than five minutes for 90% of users, making it a quick and accommodating option for busy schedules.
Conageski believes the wider implementation of this device can also benefit healthcare providers by allowing them to focus on other critical aspects of patient health during visits, knowing that home testing can be trusted.
The Future of Cervical Cancer Screening
Dr. Conageski states, "We want to ensure that a device like this meets the high reliability standards expected in-clinic. Moving forward, we will explore ways to integrate this transformative technology into healthcare systems across the nation, starting here in Colorado."

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)