Revolutionary Antioxidant Strategy Revives T Cells in Battle Against Tumors
2025-09-10
Author: Wei Ling
Tumors create hostile environments for our body’s cancer-fighting soldiers, the T cells. With low oxygen, high acidity, and various stress factors, these warriors often succumb to exhaustion, resulting in dire cancer outcomes.
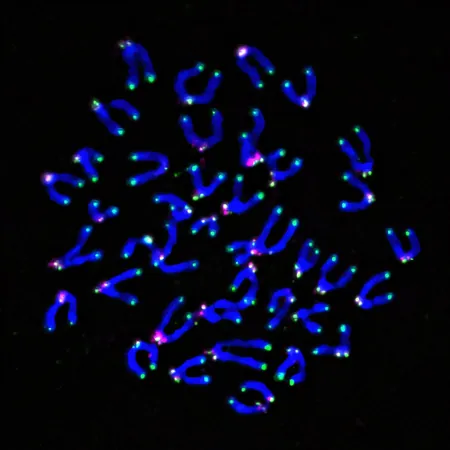
Recent groundbreaking research emerging from the University of Pittsburgh has unveiled a captivating discovery: in studies on mice, scientists found that the toxic tumor environment triggers mitochondria, the powerhouse of the cells, to unleash damaging reactive oxygen species (ROS) that harm telomeres, pushing T cells into a state of dysfunction.
Lead researcher Dayana Rivadeneira, an assistant professor in the Department of Immunology at Pitt, exclaimed, "What’s really thrilling about this research is our ability to protect telomeres with a targeted antioxidant—this can rejuvenate T cell function!" Her findings bring forth exciting prospects for innovative therapies that could enhance the effectiveness of cancer immunotherapies.
Initially, Rivadeneira and senior author Greg Delgoffe were focused on mitochondrial damage and its effects on T cells. However, a collaboration with experts Patricia Opresko and the late Marcel Bruchez redirected their focus to the implications of telomere damage.
The team engineered mice with a unique genetic system capable of producing precise oxidative damage at either telomeres or mitochondria when exposed to far-red light. Their findings were astonishing: whether they targeted mitochondria or telomeres, the outcome was the same—T cell dysfunction. Delgoffe noted, "This highlights the significant interaction between the cell’s energy producers and its control center, revealing insights previously overlooked in immunology."
Rivadeneira further emphasized, "Damage to mitochondria often leads to impaired telomeres, and conversely, damaged telomeres signal mitochondria to trigger T cell shutdown and exhaustion."
Recognizing that ROS was damaging telomeres, the researchers believed that ROS-neutralizing antioxidants could help restore T cell vitality. Their innovative approach involved tethering an antioxidant protein specifically to telomeres in mouse T cells. When infused into mice suffering from an aggressive melanoma, the results were staggering: these mice demonstrated significantly improved survival rates and smaller tumors compared to those receiving standard T cells.
The implications of this antioxidant strategy are vast, particularly for CAR-T therapy—a cutting-edge treatment that involves modifying a patient’s T cells to recognize cancer more effectively. Delgoffe remarked, "This approach could be seamlessly integrated into the CAR-T process. By enhancing T cells against cancer and safeguarding them from oxidative damage, we can bolster their effectiveness even further."
Excited about future prospects, the researchers are now focused on adapting this telomere-specific antioxidant methodology for human T cells, aiming for clinical trials down the line.
In addition to her current research, Rivadeneira intends to explore how telomere health at large impacts the immune system and cancer outcomes. One intriguing area she plans to investigate is how chemotherapy may damage telomeres and if this damage influences a patient’s responses to immunotherapy.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)