
Revolutionary Alternative to Abortion Pill Discovered in Morning-After Pill: A Game-Changer?
2025-01-24
Author: Rajesh
Introduction
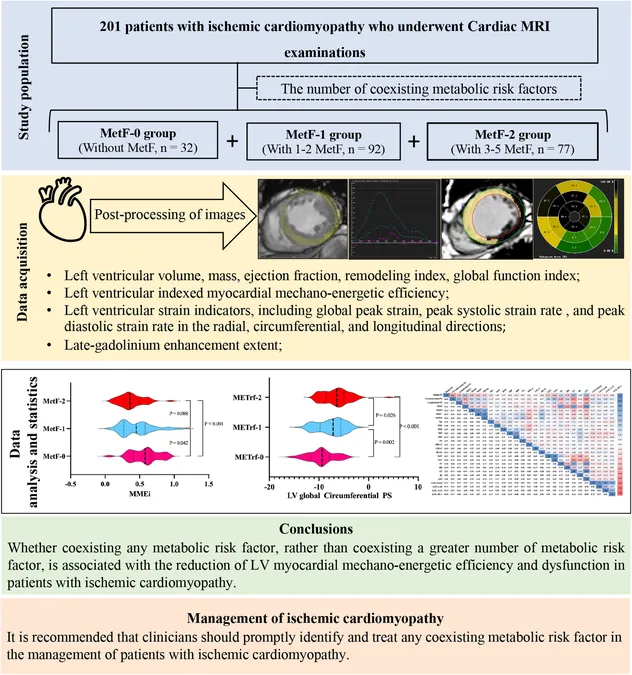
A groundbreaking study published on January 23, 2025, in the renowned journal NEJM Evidence, is stirring debates about reproductive rights and healthcare alternatives. At a time when access to mifepristone—the main abortion pill in the U.S.—is increasingly threatened by legal challenges, researchers have identified ulipristal acetate, the active ingredient in the emergency contraceptive Ella, as a potential substitute for the abortion pill regimen.
Study Details
In tests involving 133 women seeking abortion services, ulipristal acetate was administered at double the standard dose of Ella, followed by misoprostol. This new regimen achieved an impressive 97% success rate in terminating pregnancies up to nine weeks, with no serious complications reported. Most participants successfully terminated their pregnancies without the need for any additional procedures.
Legal Context
This research comes in the wake of ongoing lawsuits aimed at mifepristone, the only FDA-approved abortion pill, putting increased pressure on access to reproductive healthcare across the United States. The lead author of the study, Dr. Beverly Winikoff of Gynuity Health Projects, expressed that the Supreme Court's 2022 decision to overturn Roe v. Wade heightened her motivation to seek alternative solutions. “Maybe there’s something else we can do,” she remarked, emphasizing the importance of having readily available options on the market.
Concerns Raised
However, this study sparks concern among reproductive health experts, as it may further complicate societal perceptions of the boundary between contraception and abortion. Critics are apprehensive that it could reinforce the views of anti-abortion advocates who argue that morning-after pills can also serve as abortifacients. Despite extensive research debunking these beliefs, the new findings may provide fresh ammunition to those opposing reproductive rights.
Legal Implications
Mary Ziegler, a law professor at the University of California, Davis, cautioned that the release of this study could pose a challenge for abortion rights supporters who face increased scrutiny and debate. She highlighted how abortion opponents may interpret the study as a validation of their long-held claims regarding contraception and pregnancy termination.
Potential Challenges
Furthermore, Kristi Hamrick from Students for Life of America has already hinted at potential legal actions concerning Ella, strengthening the narrative that these organizations will pursue outcomes that align with their mission to restrict access to abortion.
Need for Caution
Despite the promising findings, experts urge caution, asserting that further research is necessary to properly assess the implications and safety of using ulipristal acetate in this new role. Dr. Kelly Cleland from the American Society for Emergency Contraception stressed that shifts in clinical practices shouldn't be based solely on this initial study. Meanwhile, Dr. Daniel Grossman from the University of California, San Francisco, has voiced his concerns over the potential ramifications for emergency contraception if higher doses of ulipristal acetate are mischaracterized as abortifacients.
Geographic Context
Conducted in Mexico City, the study raises critical questions not only about access to medication but also about the future of reproductive health policies. Dr. Paul Blumenthal, an emeritus professor at Stanford, pointed out that expanding the use of medications like ulipristal could reinforce their availability amid mounting legislative challenges.
Conclusion
As this topic continues to unfold, implications for women's reproductive rights and the battle over healthcare access remain crucial. The intersection of legal, medical, and social perspectives will undoubtedly shape the future of reproductive options in the U.S. and beyond.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)