Revolutionary AI Tool Approved by FDA to Detect Heart Disease in Chest CT Scans!
2024-11-01
Author: Rajesh
Introduction
In a groundbreaking development, HeartLung Technologies, an innovative artificial intelligence (AI) firm based in Houston, has earned the coveted 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its state-of-the-art software named AutoChamber. This new tool is set to transform the way healthcare professionals evaluate chest CT scans for signs of coronary artery disease (CAD) and other serious heart conditions that could lead to fatalities.
Features of AutoChamber
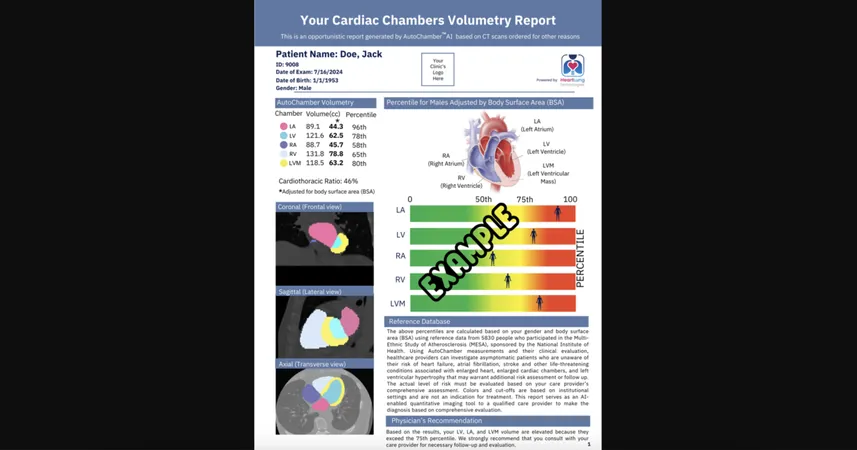
AutoChamber is equipped with advanced capabilities, enabling it to analyze various chest CT images, including those obtained for measuring coronary artery calcium (CAC) scores or for lung cancer screening. Remarkably, this AI tool can generate an insightful report within just 15 to 20 seconds, providing estimates of patient heart chamber volumes and flagging any anomalies that require clinician attention.
Integration with CCTA Scans
The use of coronary CT angiography (CCTA) scans has surged in popularity among medical teams recently, and AutoChamber seamlessly integrates with these scans, enhancing diagnostic capabilities.
Expert Opinions
Dr. Nathan Wong, a well-regarded professor of medicine and epidemiology at the University of California, Irvine, expressed confidence in AutoChamber's potential. He stated, "This tool offers a unique opportunity to evaluate cardiovascular risk for millions of individuals undergoing chest CT scans around the globe, especially those screened for lung cancer or other non-cardiac reasons. Early identification of high-risk patients could lead to timely interventions, significantly improving health outcomes."
Furthermore, Dr. Morteza Naghavi, the founder and president of HeartLung Technologies, highlighted the enhanced diagnostic power that AutoChamber brings. "By incorporating AutoChamber AI into CT scans, we can assess not only coronary disease but also risks of heart failure, atrial fibrillation, and stroke—conditions not usually reported with current scanning processes," he said, emphasizing the vast potential when applied to millions of lung CT scans performed daily.
Regulatory Approval and Future Outlook
The FDA had previously granted AutoChamber breakthrough device designation, indicating an acknowledgment of the technology's significant potential from the outset. This program aims to expedite the approval process for medical devices, allowing them to reach patients faster. The FDA's proactive collaboration with HeartLung Technologies to prioritize submissions related to this device is a testament to its promise in advancing cardiac care.
Conclusion
As the healthcare community eagerly anticipates the widespread adoption of AutoChamber, this revolutionary tool signifies a major leap forward in leveraging artificial intelligence for early detection and improved management of heart disease. With the integration of such advanced technology, the healthcare landscape is poised for a transformative change in how cardiac risks are evaluated and addressed.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)