Revolutionary AI Technique Transforms Microscopy: Get Crystal-Clear Images of Thick Biological Samples!
2025-01-28
Author: Daniel
Depth degradation is a well-known challenge in the field of biology. As researchers probe deeper into samples, like tiny worm embryos or thin tissue fragments, the clarity of microscopic images diminishes due to the scattering and bending of light. While advanced techniques like adaptive optics have been developed to overcome this issue, they often come with high costs, additional equipment, and the need for specialized knowledge, making them inaccessible to many research facilities.
But a groundbreaking advancement from the Shroff Lab at the Janelia Research Campus, part of the Howard Hughes Medical Institute (HHMI), could change everything. The team has unveiled an innovative AI-driven method that allows researchers to produce sharp, high-quality microscopy images throughout thick biological samples—without the need for fancy hardware or complex setups.
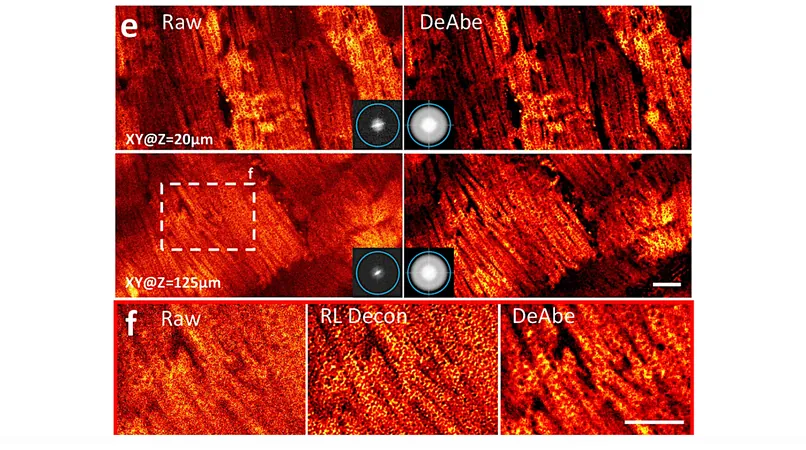
By developing a model to understand the degradation of images as they penetrate deeper into a uniform sample, the researchers created a transformative technique. They took clear images from the surface of a sample, applied artificial distortions to mimic deeper captures, and then trained a neural network to reverse that distortion. The result? Remarkably clear images that retain their quality throughout the entire depth of the sample.
This pioneering deep learning method doesn't require sophisticated adaptations; all users need is a standard microscope, a computer with a decent graphics card, and a brief tutorial on the software. This makes it an accessible alternative for biology labs worldwide, democratizing high-resolution imaging.
Not only does this technique enhance image quality, but it also improves the accuracy of biological assessments. Researchers reported significant strides in their ability to count cells in worm embryos, trace intricate blood vessels and structures in mouse embryos, and examine the mitochondria of various tissues in mouse livers and hearts with unprecedented detail.
The Shroff Lab is already harnessing this advanced technology for imaging worm embryos, but their aspirations don't stop there. The team is planning to refine their model further to accommodate a wider variety of samples, including those that are less uniform and more complex in structure.
Imagine the possibilities this technology could unlock in the world of biomedical research! From cancer studies to regenerative medicine, clearer imaging could lead to major breakthroughs. As this AI technique continues to evolve, it promises to become an essential tool for biologists striving to unravel the complexities of life at a microscopic level.
Stay tuned as we uncover more captivating developments in the world of microscopy and artificial intelligence—this could very well be the future of biological imaging!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)