Revolutionary 3D-Printed Device Transforms Human Tissue Modeling in the Lab
2025-05-24
Author: Wei
Breaking Ground in Tissue Engineering
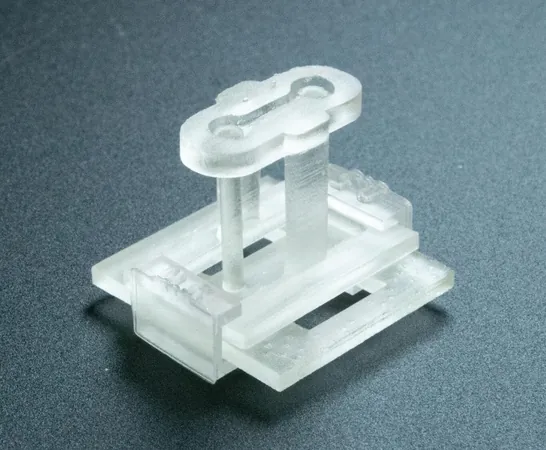
A groundbreaking 3D-printed device is set to revolutionize the way scientists model human tissues, bringing unprecedented control and complexity to biomedical research. Developed by an innovative team at the University of Washington and UW Medicine, this device, known as STOMP (Suspended Tissue Open Microfluidic Patterning), paves the way for advancing therapies targeting various diseases.
The Quest for Realistic Lab-Made Tissues
The primary aim of tissue engineering is to replicate natural cell habitats within the lab, simulating the intricate environments cells thrive in. Traditional methods often involve suspending cells in gels between freestanding posts to cultivate heart, lung, skin, and musculoskeletal tissues. While this has allowed cells to behave more like they would in the human body, it hasn't fully tackled the challenge of studying multiple tissue types simultaneously.
Unlocking the Complexities of Human Biology
STOMP equips researchers with the tools necessary to gain precise control over tissue composition and arrangement. This leads to better modeling of complex diseases, including neuromuscular disorders. A recent study published in *Advanced Science* reveals how STOMP enables scientists to assess cell responses to mechanical cues while creating distinct regions in suspended tissues.
From Concept to Creation: The Technology Behind STOMP
Professors Ashleigh Theberge and Nate Sniadecki spearheaded the development, demonstrating how STOMP can effectively recreate biological interfaces such as bone and ligament, as well as fibrotic and healthy heart tissues. By enhancing a tissue-engineering method akin to molding Jell-O, STOMP utilizes capillary action to arrange different cell types precisely within the gel—a significant leap in biomedical engineering.
Real-World Applications and Experiments
In action, STOMP has already been tested in two notable experiments: one evaluating the contractile dynamics of both healthy and diseased heart tissues, and the other simulating the ligaments connecting teeth to bone sockets. This fingertip-sized device integrates with systems designed for measuring heart cells' contractile force, providing intricate control over tissue design without the need for cumbersome additional equipment.
Innovative Features Drive Versatility
Equipped with degradable walls thanks to hydrogel technology from the DeForest Research Group, STOMP allows for the easy breakdown of device sides while keeping the tissue intact. "Cells have a tendency to pull tissues together, often leading to shrinkage from mold walls. For weaker cells or certain biomaterials, this design provides invaluable versatility," Sniadecki explained.
The Future of Tissue Engineering is Here
Theberge is enthusiastic about the potential of STOMP, stating, "This method opens new frontiers in tissue engineering and cell signaling research. It was a true collaborative effort from multiple disciplines, and we can’t wait to see how it will be employed in future studies." As the boundaries of medical science continue to expand, innovations like STOMP hold the key to unlocking the mysteries of human biology.







 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)