Revealing Triptorelin's Hidden Risks: A Deep Dive into Adverse Events
2025-09-01
Author: Yu
Unmasking Triptorelin: The Gonadotropin-Releasing Hormone Game Changer
Triptorelin, a pivotal synthetic analogue of gonadotropin-releasing hormone (GnRH), has been a monumental player in hormone therapy since its inception by Schally in 1973. With its unique D-tryptophan modification, this powerful drug binds more effectively to receptors, leading to enhanced activation and a longer-lasting presence in the bloodstream compared to natural GnRH. Its influence is profound, initially stimulating pituitary secretion before ultimately suppressing sex hormone production, akin to a castrating effect.
In practice, Triptorelin serves critical roles in male patients, primarily curbing testosterone levels to combat prostate cancer, while in females, it halts ovarian estrogen production, proving vital for conditions like endometriosis. Children with central precocious puberty (CPP) also benefit from Triptorelin, as it effectively halts premature hormonal activation.
The Balancing Act: Benefits vs. Risks
However, the advantages of Triptorelin come with a spectrum of potential adverse reactions. The safety profile, influenced heavily by its endocrine effects, has led to numerous documented adverse reactions, notably hot flashes—which a staggering 70% of users report. Male patients often grapple with sexual dysfunction and acute tumor flare-ups, while females report gynecological issues like unusual bleeding and mood fluctuations. Notably, children exhibit unique reactions, including growth changes and allergic responses.
Harnessing Data: Insights from FAERS
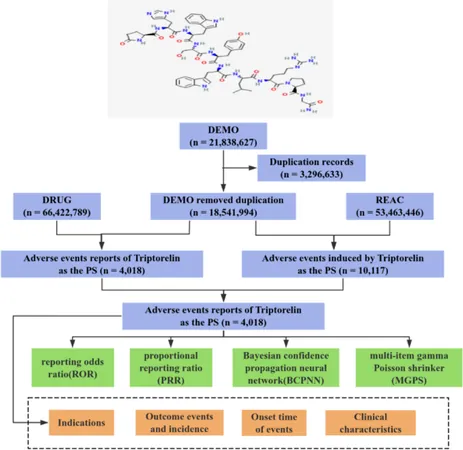
To better understand these risks, a comprehensive analysis of the FDA’s Adverse Event Reporting System (FAERS) was conducted, examining data from 2004 to 2024. This resource is instrumental in tracking spontaneous reports of adverse drug events, providing a rich tapestry of real-world safety signals not always captured in clinical trials.
In total, over 21 million reports yielded 10,117 adverse events specifically linked to Triptorelin. Gender distribution among these reports was nearly equal, while age discrepancies revealed heightened adverse event reports among both older adults and younger patients, underscoring the importance of tailored monitoring.
Uncovering New Signals and Serious Risks
Through rigorous statistical analysis, the study identified over 70 significant safety signals. Many were expected, aligning with known side effects of the drug, but strikingly, new associations surfaced, such as links to Alzheimer’s disease and defiant behavior in children. These findings illuminate pressing areas for further research, raising questions about whether these correlations stem from the drug itself or underlying health conditions.
The Time Factor: When Do Adverse Events Strike?
The analysis also tackled the timeline of adverse reactions, revealing that while many occur within the first month of treatment, others arise unexpectedly long after therapy initiation. This highlights the critical need for ongoing monitoring and timely intervention.
Navigating the Gender Divide in Adverse Reactions
Gender disparities in reported adverse events emerged as another crucial dimension. Females were notably more prone to specific adverse effects, such as abdominal pain and psychiatric disorders, while males reported higher occurrences of fatigue and medical complications due to their underlying conditions.
Towards A Safer Future: Recommendations for Healthcare Providers
Given these insights, the need for heightened vigilance among healthcare providers is clear. Continuous monitoring and timely response to both established and emerging adverse events must be prioritized to ensure patient safety. Future studies should focus on identifying potential confounders and establishing clearer causal relationships. As Triptorelin continues to be a cornerstone in hormone therapy, understanding its full impact is essential for optimizing treatment and improving outcomes for all patients.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)