Revealing the Gaps: A Deep Dive into Data Sharing Practices in Clinical Trials
2025-09-01
Author: Mei
Unveiling Clinical Trials: Data Sharing in the Spotlight
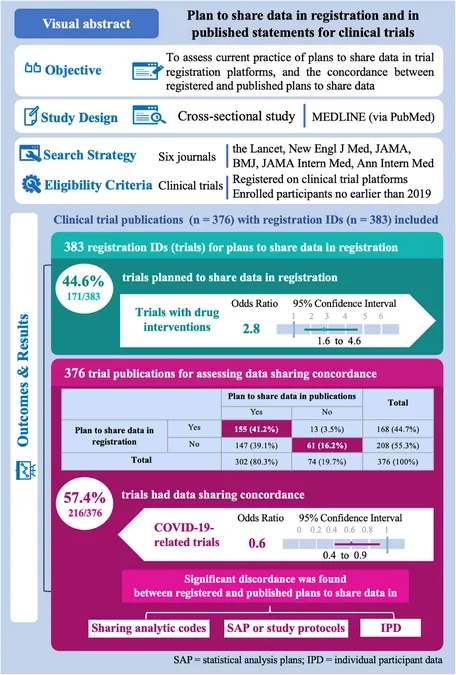
A groundbreaking study has cast light on the critical yet often overlooked practice of data sharing in clinical trials, scrutinizing how well these intentions are reflected from registration to publication. The findings are alarming: over 40% of clinical studies show discrepancies between what they promise in terms of data sharing and what they actually deliver.
The Methodology: Selecting the Right Trials
The research analyzed data from six prestigious medical journals known for publishing clinical trials, including The Lancet and the New England Journal of Medicine. Focusing on studies registered from 2019 onwards, when the International Committee of Medical Journal Editors (ICMJE) mandated data sharing plans, the analysis encompassed 376 trials conducted across various medical fields.
Key Findings: Data Sharing Practices and Their Discrepancies
Out of the trials examined, a staggering 44.6% reported a plan to share data during registration, yet many failed to uphold these commitments in their subsequent publications. This discordance highlights a significant gap in the credibility of clinical trial reporting.
Drug trials were notably more likely to include robust data sharing plans, with an odds ratio of 2.71 compared to non-drug trials. In contrast, those related to COVID-19 showed reduced odds of conformity, raising questions about the transparency of pandemic-related research.
Unpacking the Word Gap: Understanding Disparities in Data Plans
The study delved deeper into what specific data sharing plans were communicated. While there was an increase in trials intending to share individual participant data (IPD) from registration to publication, the sharing of statistical analysis plans (SAPs) plummeted from 91 to merely 43 trials. This suggests a worrying trend where trials may be overstating their commitment to data transparency.
A Call for Improved Compliance and Transparency
Despite the clear guidance issued by the ICMJE, over 40% of trials exhibited discordance in their data sharing plans. The study advocates for a re-evaluation of these requirements to ensure that registration reflects actual practices more accurately.
Moreover, the findings raise broader questions about the challenges trial authors face, including potential conflicts of interest, participant privacy concerns, and the resources needed to manage data sharing responsibly.
Conclusion: The Imperative for Change in Clinical Trials
The study's implications could reshape the landscape of clinical trial transparency. As calls for data access gain traction, the research underscores the pressing need for stricter adherence to data sharing plans and a cultural shift towards accountability in how trial data is managed and reported.
As the medical community grapples with these findings, it is evident that fostering a culture of transparency isn't just beneficial—it's necessary for the integrity of clinical research.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)