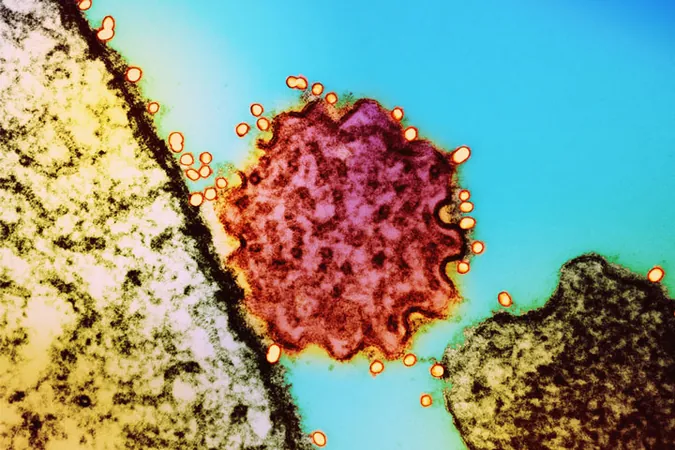
Nipah Virus Vaccines Poised for Human Trials Amid Rising Threats
2025-07-15
Author: Mei
Human Trials on the Horizon for Nipah Vaccines
In a groundbreaking development, two vaccines targeting the deadly Nipah virus—transmitted by bats—are slated to enter human clinical trials in Bangladesh. This comes as new drug candidates show promise not only in treating but also in preventing infections.
A Looming Epidemic?
Although Nipah cases remain limited, increasing climate change is altering ecosystems, leading to closer contact between humans and fruit bats, the virus's primary carriers. Alarmingly, around 2 billion people are considered at risk, highlighting the urgent need for effective vaccination.
The recent Nipah outbreak in Kerala, India, where health officials confirmed two cases—one fatal—has further intensified the urgency for new vaccines. Over 400 individuals are currently being monitored for signs of the virus.
Why Nipah is So Dangerous
Nipah virus boasts a staggering fatality rate of up to 75%. The World Health Organization (WHO) has labeled it a priority pathogen capable of causing widespread outbreaks. Even non-fatal infections leave victims with severe, long-lasting effects; months after previous outbreaks, two individuals in India remain in comas.
Bangladesh and India: The Epicenter of Nipah Outbreaks
Bangladesh and India have recorded the highest incidence of Nipah virus cases. The virus is primarily spread through contact with bat droppings and contaminated substances, often transferring from person to person through respiratory droplets. Initial symptoms resemble acute respiratory infections, advancing quickly to severe complications including encephalitis.
Innovative Vaccine Development
At the forefront of vaccine development is the ChAdOx1 NipahB vaccine, created by the University of Oxford and based on the same platform used for the AstraZeneca COVID-19 vaccine. Recently sanctioned by the European Medicines Agency's PRIority MEdicines (PRIME) scheme, this development is crucial for expediting regulatory approvals.
Professor Brian Angus, head of the trials, emphasized the significance of this support in accelerating the regulatory approval process. With promising results from animal studies and ongoing Phase 1 trials, the vaccine has the potential to be a major breakthrough.
Emergency Use Approval: A Glimmer of Hope
Vaccines still under investigation can gain emergency use authorization during outbreaks, akin to the Ebola vaccine rollout during its crisis. Dr. Nicole Lurie from CEPI noted that

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)