New Study Unveils Groundbreaking Mechanism of mRNA Poly(A) Tail Regulation in Early Embryos
2025-01-14
Author: Jia
Introduction
In a transformative investigative breakthrough, researchers from the Chinese Academy of Sciences have uncovered a critical mechanism governing mRNA poly(A) tail regulation during the pivotal oocyte-to-embryo transition (OET). This regulation directly influences the translational efficiency of maternal mRNA, which is vital for selective protein translation in developing early embryos.
Key Findings
Published in Nature Communications on January 2, the collaborative study reveals that the poly(A) tail length plays an essential role in mRNA stability and translation during the crucial moments of embryonic development. However, the specific mechanisms behind this regulation have long been shrouded in mystery—until now.
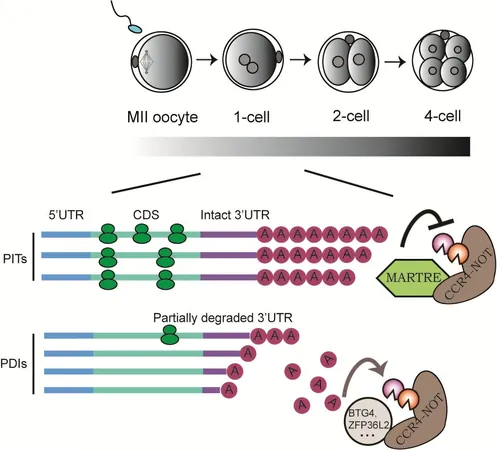
The research team identified a key player in this complex process: a poly(A) tail regulatory factor named MARTRE1. This factor is selectively expressed in a specialized cell state known as 2-cell-like cells (2CLCs). Through a series of cellular and biochemical experiments, the team demonstrated that MARTRE1 acts as an inhibitor of the poly(A) deadenylase complex CCR4-NOT, which is responsible for the shortening of mRNA poly(A) tails. By inhibiting this complex, MARTRE1 was able to slow the rate of poly(A) tail shortening significantly, thereby enhancing mRNA stability and translating efficiency.
Gene Family Discovery
Interestingly, the researchers discovered that the Martre1 gene is part of a previously under-explored family of genes now designated as the Martre gene family. Further investigation involved creating a mouse model that lacked the entire Martre gene family. The result? A noticeable delay in early embryonic development among the knockout mice, underscoring the critical role of MARTRE proteins.
Methodologies Used
Employing advanced methodologies such as transcriptomics, translatomics, and third-generation sequencing, the researchers meticulously measured the lengths of mRNA poly(A) tails. Their findings confirmed that MARTRE proteins are instrumental in safeguarding long-tailed mRNAs from excessive deadenylation during the early stages of embryonic development. This protective mechanism is crucial for ensuring that maternal mRNAs are efficiently translated, allowing the embryo to thrive even with limited maternal resources.
Implications and Future Directions
Prof. Bing Zhu, who led the study, commented on the implications of these findings, stating, "By protecting translated mRNAs from deadenylation, the early embryo can sustain efficient translation using limited maternal mRNA, which may represent a universal strategy for regulating maternal gene translation during early development across various species."
This pivotal research not only provides new insights into the intricate control of early embryonic development but also opens up exciting avenues for further exploration in developmental biology and reproductive medicine. What other secrets might still be lurking in the world of embryonic regulation? Stay tuned as science continues to unravel the mysteries of life!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)