New ESAT6-CFP10 Skin Test Outperforms Tuberculin Test: Groundbreaking Study Unveils Surprising Results!
2024-12-30
Author: Wei
Introduction to a Significant Study
A recent clinical trial has sparked a wave of excitement among health professionals as it evaluated the effectiveness of the ESAT6-CFP10 (EC) skin test, designed to diagnose tuberculosis (TB). Conducted in a healthy population, this extensive phase III randomized controlled study shed light on an innovative method of TB screening that could revolutionize public health strategies.
Study Overview and Methodology
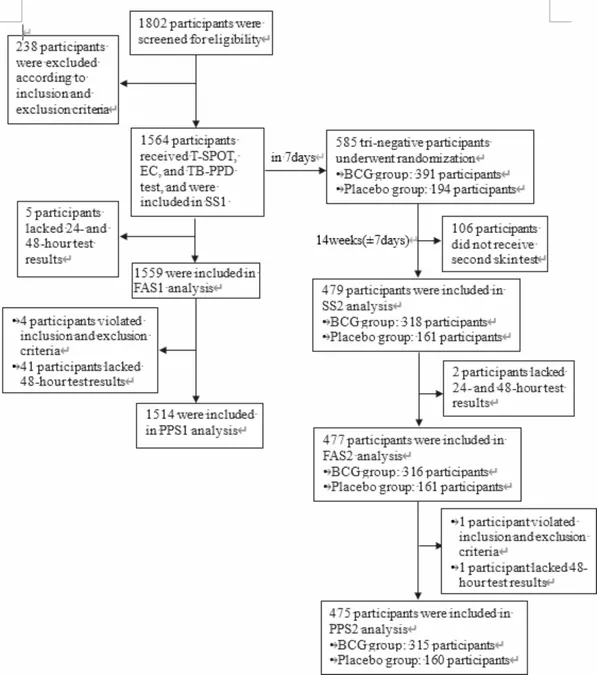
This comprehensive study aimed to compare the diagnostic performance of the EC skin test with that of the traditional tuberculin skin test (TB-PPD) among healthy individuals, exploring factors influencing the skin test responses. Researchers gathered a total of 1,564 participants from Jiangsu Province, China, conducting a randomized, double-blind, parallel controlled trial. In the first stage, all subjects underwent three tests: T-SPOT, TB-PPD, and the EC skin test.
Following the initial assessment, participants who yielded negative results from all three tests were further analyzed in the second stage. They were randomly assigned to receive either the BCG vaccine or a placebo to determine if previous TB vaccination influenced the effectiveness of the new skin tests.
Key Findings That Could Change TB Testing
The results were revealing. In the first stage, positivity rates were documented as follows: T-SPOT showed a positivity rate of 18.89%, EC had a rate of 10.37%, and TB-PPD had a staggering 53.96%. Notably, the EC skin test demonstrated an impressive concordance of 88.97% with T-SPOT, which indicates a strong correlation between the two testing methods.
As the study progressed, participants in the BCG group displayed varying positivity rates for the T-SPOT, EC, and TB-PPD tests, revealing the nuanced effects of the vaccine on test outcomes. Notably, age (specifically individuals aged 60 and over) and retest positivity of the T-SPOT test emerged as influential factors in the EC's booster effect.
Safety Observations Provide Reassurance
Despite some participants experiencing mild adverse reactions (primarily localized irritation), the study concluded that the EC skin test was safe for use. Only 10.74% experienced side effects, with most cases involving minor issues such as site pain or rash. No serious adverse events linked directly to the EC test were reported, reinforcing its potential as a viable external TB screening tool.
Broader Implications for TB Control
The findings support EC as a promising new instrument for TB testing, especially among groups with high exposure risk or compromised immunity. Given the global push to eradicate TB, the adoption of more specific and reliable testing methods like EC could help advance the World Health Organization’s goal to end the tuberculosis epidemic by 2030.
The Road Ahead: Future Research and Recommendations
While the trial showcased the EC test's reliability and safety, questions remain regarding the booster effect's underlying mechanisms and the influence of environmental factors on testing outcomes. Future research should aim to clarify these aspects, ensuring the most effective TB control strategies can be implemented.
Conclusion: A New Era for Tuberculosis Diagnosis?
In conclusion, this study is not just a step forward in tuberculosis diagnosis; it signifies a potential transformation in how we approach TB screening on a global scale. With the EC skin test showing equal or better performance than the traditional methods, and exhibiting satisfactory safety, it may lead to improved TB detection efforts and ultimately save lives—ushering in a new era in public health, where precision testing could become the standard in combating tuberculosis.
Stay tuned as we continue to unravel the many implications of this groundbreaking research!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)