New Discoveries in Cellular Quality Control: How Two Proteins Guard Against Defective Protein Blueprints
2025-03-28
Author: Sarah
In a significant breakthrough in cellular biology, researchers have uncovered the critical roles of two molecular control factors involved in the process of splicing, a fundamental step in the assembly of mature messenger RNA (mRNA) essential for protein synthesis. This pioneering study, led by Prof. Dr. Ed Hurt of the Heidelberg University Biochemistry Center, in collaboration with teams from Fudan University in Shanghai and the Max Planck Institute for Multidisciplinary Sciences in Göttingen, has been published in the esteemed journal *Cell Research*.
Proteins, the building blocks of life, perform crucial functions within organisms, and their synthesis relies on precise genetic instructions embedded in DNA. To produce proteins, cells first transcribe this information into precursor mRNA (pre-mRNA), which, intriguingly, contains both coding sequences (exons) and non-coding segments (introns). The effective splicing of pre-mRNA, which involves excising introns and connecting exons, ensures that only the necessary information for protein production is maintained.
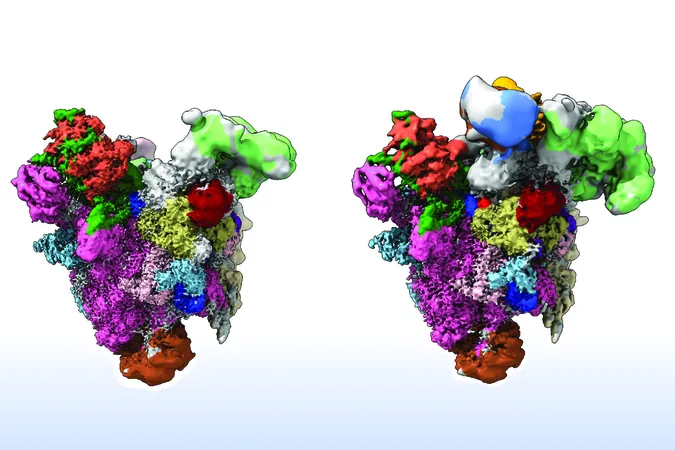
At the heart of splicing is the spliceosome, an intricate molecular machine comprised of numerous proteins and RNA components. This machine must accurately identify and cut specific exon-intron junctions, a task which is vital for producing functional mRNA.
Despite extensive knowledge about how the spliceosome operates, the mechanisms through which it can identify and reject faulty precursor mRNA remain poorly understood. The new research sheds light on this mystery, with findings indicating that two key proteins—GPATCH1 and DHX35—serve as essential quality control agents during splicing.
Utilizing spliceosomes from the filamentous fungus *Chaetomium thermophilum*, the researchers demonstrated that GPATCH1 and DHX35 play pivotal roles when defective pre-mRNA is detected. GPATCH1 acts as a sensor, signaling that the spliceosome should halt its activity when a problem is identified, while DHX35 physically removes the unsuitable pre-mRNA and disassembles the spliceosome back into its components, ready for the next round of splicing.
Dr. Paulina Fisher, a postdoctoral researcher involved in the study, emphasized the importance of these findings: “When faced with defective pre-mRNA, these proteins swiftly mobilize to ensure quality control, preventing erroneous protein synthesis from improperly spliced mRNA.”
The implications of this research extend beyond basic biology. An enhanced understanding of the splicing quality control mechanisms could lead to new therapeutic strategies for diseases caused by splicing errors, including various forms of cancer and genetic disorders. Scientists are optimistic that further exploration of these molecular inspectors will unravel additional layers of complexity within gene expression regulation.
As we continue to dive deeper into the cellular machinery that governs life at a molecular level, the discoveries concerning GPATCH1 and DHX35 mark an exciting step toward comprehending the intricacies of genetic fidelity, offering promising avenues for future medical advancements.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)