Navigating the Complex World of Microbial Pesticides: Risks and Regulatory Challenges in the EU
2025-07-02
Author: Wei
The European Green Deal and Microbial Pesticides
The European Green Deal stands at the forefront of the EU's commitment to climate policy, aiming to make food and production systems not only sustainable but also protective of human health and the environment. Central to this initiative is the Farm2Fork Strategy, which seeks to cut the use of hazardous pesticides by 50% by 2030, promoting biological alternatives and combating antimicrobial resistance (AMR). However, the journey toward achieving these ambitious goals highlights the crucial role of biopesticides, particularly those rigorously evaluated within the EU's stringent regulatory framework.
Defining Biopesticides: A Complex Landscape
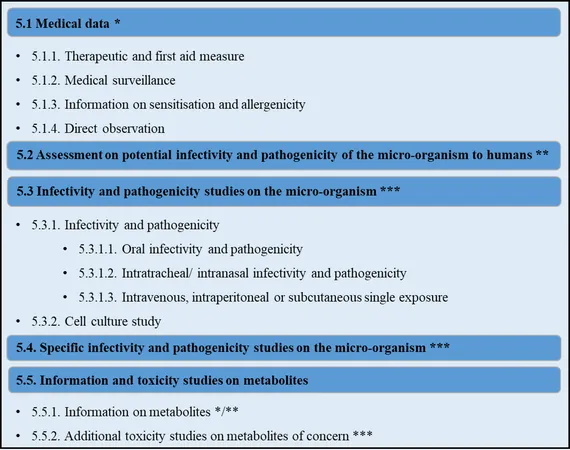
The term 'biopesticide' lacks a unified definition, encompassing a wide range of biologically derived substances—from microorganisms and their metabolites to plant extracts. Additionally, innovative methods like RNA interference and microbial consortia are emerging. In the EU, the approval of microbial pesticides entails a meticulous evaluation process that assesses factors like pathogenicity, infectivity, and the presence of AMR genes.
Challenges in Assessing Human Health Risks
Assessing risks associated with microbial active substances (AS) involves a literature review and a weight of evidence approach to determine potential threats to human health. However, demonstrating safety is complicated; animal studies may not provide conclusive data regarding human risks due to differences in host specificity and the nuances of microbial behavior.
The Need for Updated Testing Strategies
Current testing guidelines for biopesticides are nearly three decades old, necessitating new testing strategies that align with modern science. Concepts like Integrated Approaches to Testing and Assessment (IATA) could pave the way for improved risk assessment, especially by leveraging findings from initiatives like those developed during the COVID-19 pandemic.
Innovative Approaches: New Methodologies for Risk Assessment
Emerging tools and methodologies, such as New Approach Methodologies (NAMs) emphasize mechanism-driven assessments over traditional toxicological endpoints. These advancements promote the use of tiered testing approaches and aim to reduce the reliance on animal models—a significant ethical concern in contemporary research.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)