Nature's Own Secret: How a Unique Protein Thrives in Oxygen While Producing Hydrogen!
2025-01-15
Author: Ming
Introduction
Researchers from Bochum, Germany, and Osaka, Japan, have made a groundbreaking discovery about a rare protein that can produce hydrogen, even in the hostile presence of oxygen. While most hydrogen-producing enzymes, known as [FeFe]-hydrogenases, are vulnerable to damage from oxygen, a particular enzyme called CbA5H, derived from the bacterium Clostridium beijerinckii, appears to defy this norm.
The international team, led by Professor Thomas Happe of Ruhr University Bochum and Professor Genji Kurisu from Osaka University, published their findings in the *Proceedings of the National Academy of Sciences*. Their discovery sheds light on the unique molecular mechanisms that allow CbA5H to survive oxygen exposure, a key factor needed for improving large-scale hydrogen production, which is vital for sustainable energy solutions.
Unlocking the Mysteries with Advanced Imaging Techniques
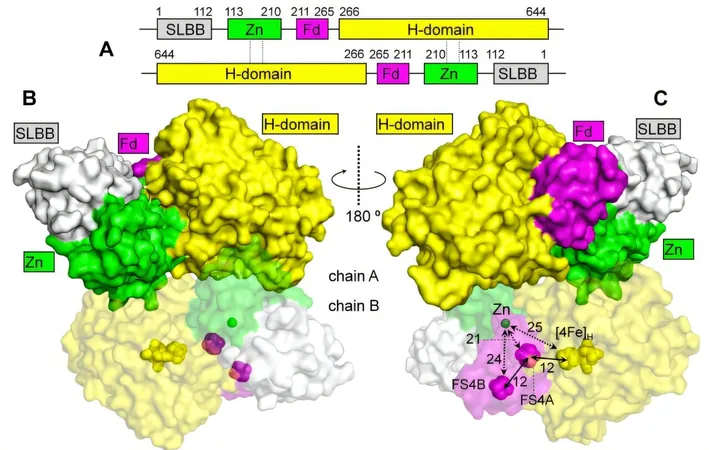
Three years prior, researchers had only partial knowledge of CbA5H’s structure under oxygen-rich conditions, revealing that its active site was somewhat protected by a nearby sulfur group. However, the exact process by which it produced hydrogen remained unclear. By utilizing state-of-the-art cryo-electron microscopy (cryoEM), they achieved a complete structural analysis of CbA5H in an oxygen-free (anoxic) environment, providing deeper insights into its functionality.
The newly acquired structure illustrated that the sulfur-containing protective group detaches from the active center, establishing the true hydrogen-producing condition of CbA5H. "By comparing the structures obtained in both oxygen-rich and oxygen-free environments, we could successfully discern its operational and protection strategies against oxygen," explained Jifu Duan, a key researcher on the project.
The Role of Zinc in Stability and Functionality
In addition to unraveling the O2 protection mechanisms, the complete structural analysis revealed another critical aspect of CbA5H: its formation of a homodimer, a complex formed by two identical CbA5H molecules. This dimerization is crucial for its stability and functionality, supported by a zinc (Zn2+) binding motif. The research highlighted that the Zn2+-mediated homodimer is far more stable than its monomeric counterpart, especially when modifications remove Zn2+.
This finding underscores the potential of utilizing CbA5H in future bioengineering applications for hydrogen production. As the world seeks renewable energy solutions, understanding and harnessing such extraordinary proteins could pave the way for breakthrough technologies in hydrogen fuel production.
Conclusion
In summary, the collaboration between German and Japanese scientists has provided valuable insights into how nature's own mechanisms can inspire sustainable energy solutions. Keep your eyes peeled for more developments in this rapidly advancing field!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)