Mpox Declared International Emergency: What You Need to Know About the Rising Threat
2024-10-01
Mpox Declared International Emergency
The World Health Organization (WHO) has officially declared mpox, previously known as monkeypox, a public health emergency of international concern (PHEIC). This alarming response comes amidst a sharp increase in cases that some experts liken to the initial days of the HIV epidemic due to the virus's severity, uncertainties surrounding it, and the stigma that accompanies its transmission.
Importance of Declaration
This declaration paves the way for expedited access to critical resources such as testing, vaccines, and medications in regions heavily impacted by the outbreak. The WHO has already allocated $1.5 million from its contingency fund to assist containment efforts, particularly in the Democratic Republic of Congo (DRC) and its neighboring countries, which are experiencing an outbreak caused by a newly identified variant of the virus—clade IB.
Emergence of New Strain
This new strain emerged in January 2023 and has already spread to 12 additional countries, raising concerns due to its deadlier nature compared to the previously circulated clade II during the 2022 outbreak, which resulted in approximately 100,000 cases worldwide.
Challenges in Treatment
Currently, the medical community is facing significant challenges, as there is no specific treatment approved for mpox infections. Management of the illness largely revolves around supportive care for symptoms like pain and fever. Antiviral treatments, such as cidofovir and tecovirimat, are occasionally employed, having been previously approved for smallpox, although their effectiveness and long-term impact on mpox remain uncertain.
Need for Vaccination
The urgent need for a vaccine cannot be overstated, as it is expected to be a vital tool in controlling the spread of mpox.
Vaccine Innovation in China
In a promising development, China is at the forefront of vaccine innovation against mpox. The Shanghai Institute of Biological Products has developed a vaccine candidate based on a live, attenuated strain known as MVA, which was granted approval to enter clinical trials on September 9.
Clinical Trials and Research Efforts
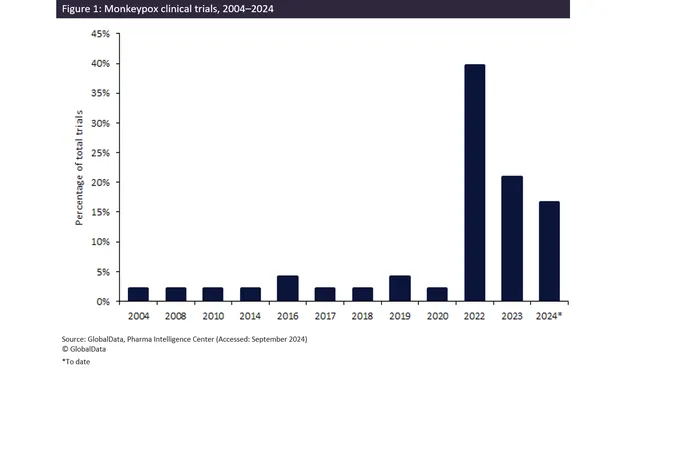
While the number of clinical trials focused on smallpox remains higher than those for mpox, the interest is gradually picking up. GlobalData’s trials intelligence platform indicates a surge in mpox-related trials in 2022 due to heightened attention following previous outbreaks, even though participation dipped in 2023.
Future Outlook
Experts predict that these numbers could anticipate another surge, especially as pharmaceutical companies respond to the latest wave of infections in 2024. With research efforts having increased by 88% since 2004, the global health community and pharmaceutical sector must continue to ramp up initiatives to effectively combat this growing threat.
Conclusion
As the situation evolves, staying informed and vigilant is crucial. The latest data suggests that without swift action and innovation, the challenge posed by mpox could escalate, raising serious public health concerns worldwide.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)