Maternal Echinococcus Multilocularis Infection: A Surprising Shield Against Colitis in Mice
2025-07-26
Author: Wei
The Helminth Infection Enigma
Helminth infections touch the lives of over 1.5 billion people worldwide, posing a chronic threat to public health. However, intriguingly, these parasitic infections seem to correlate inversely with autoimmune diseases like allergies and inflammatory bowel disease (IBD). This paradox raises essential questions about the immune-modulating capabilities of helminths.
A New Perspective on Autoimmunity
Recent research suggests that helminths may offer a groundbreaking approach to managing autoimmune diseases. They primarily influence the host's immune system via Th2 responses and expansion of regulatory T (Treg) cells, crucial for maintaining immune balance and curbing inflammation. A vital player in this regulation is Foxp3, a transcription factor essential for Treg functionality. Furthermore, helminth infections can significantly alter the gut microbiome, emphasizing the interconnectedness of gut health and immune responses.
Maternal Influence on Gut Health
The gut microbiota, which develops early in life—largely through maternal transmission—plays a pivotal role in health. Recent studies unveil that changes in maternal gut microbiota due to helminth infections can benefit offspring. For instance, infected mothers might impart a microbiome that offers resistance to obesity and potentially shields against conditions like colitis.
Exploring Echinococcus Multilocularis in Offspring
In this groundbreaking study, researchers examined the influence of maternal Echinococcus multilocularis (Emu) infection on the offspring's gut health and immune responses. Using a mouse model, the research aimed to unravel how maternal Emu infection modulates gut microbiota and impacts colitis susceptibility in young mice.
Impact of Emu Infection: A Closer Look
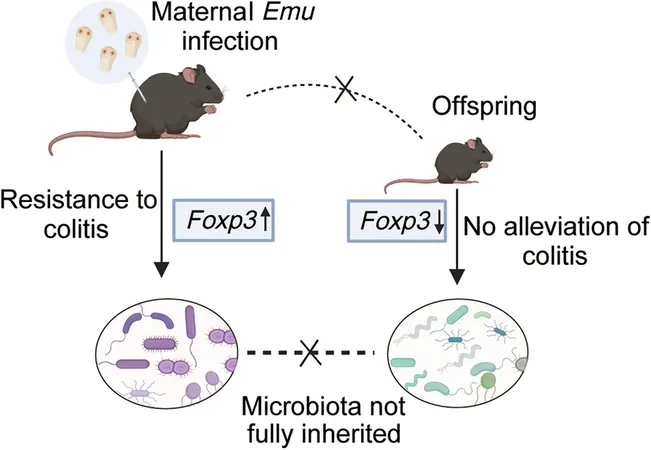
Infected maternal mice subjected to colitis via dextran sodium sulfate (DSS) displayed significantly reduced disease severity compared to uninfected mice. They experienced lower disease activity index (DAI) scores, less weight loss, and preserved colon length—demonstrating that maternal Emu infection confers a protective advantage against intestinal inflammation.
Offspring Outcomes: A Disappointing Twist
Contrary to expectations, the offspring of Emu-infected mothers failed to inherit these protective benefits. They exhibited significant weight loss and severe colonic damage when exposed to DSS, mirroring the outcomes of offspring from uninfected mice. This indicates that maternal immunity induced by Emu infection does not transfer to the next generation.
Unpacking the Immunological Mystery
Hierarchy within immune regulation became apparent as the study found that elevated Foxp3 levels seen in maternal mice did not translate to their offspring. The lack of a significant increase in Foxp3 expression in young mice suggests that inherited immune advantages are not a given.
Trekking Through the Microbiota Landscape
Analysis of gut microbiota revealed significant changes due to maternal Emu infection, particularly in microbial composition. While maternal gut profiles favored beneficial bacteria, offspring displayed distinct patterns. Genera linked to inflammation increased in young mice, indicating that protective microbiota traits may not be effectively passed down.
Conclusion: A Call for Further Investigation
This study opens the door to new inquiries regarding the complexities of maternal infection, microbial transmission, and autoimmune disease prevention. Despite interesting findings, limitations such as reduced sample sizes and incomplete understanding of precise mechanisms warrant additional research. If we can uncover how maternal factors influence microbiota and immune responses, we may pave the way for innovative treatments for conditions like IBD.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)