Journey of Viruses: How Insects Like Mosquitoes Become Disease Vectors
2024-09-24
A groundbreaking study involving fruit flies has deciphered the intricate pathways viruses take within insects to ultimately infect new hosts through bites. This research sheds light on vital mechanisms at play, particularly how mosquitoes and similar insects serve as vectors for viral diseases such as dengue, Zika, chikungunya, and yellow fever.
Understanding how viruses traverse different tissues within an insect opens the door to innovative solutions. Researchers envision the possibility of genetically engineering mosquitoes that cannot transmit these deadly viruses, thereby controlling outbreaks by introducing these modified insects into wild populations to mate and alter their genetic makeup.
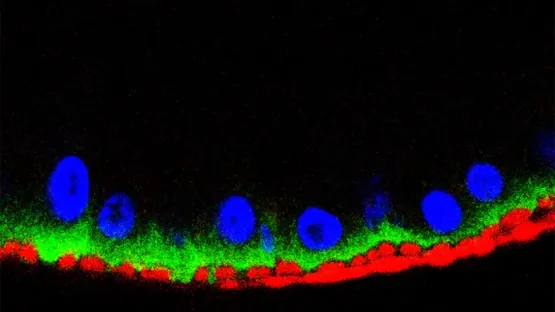
To transmit viruses, mosquitoes must first ingest them. The virus then navigates through the insect’s midgut epithelial cells, gains entry into the circulatory system, and exits via salivary glands. It is during this feeding process that the virus enters a new host, poised to spread disease.
Scientists have long been puzzled by how a virus can enter through one type of cell and exit through another, particularly since these processes require distinct mechanisms. Co-senior author Nicolas Buchon, an associate professor of entomology, emphasized the significance of this knowledge for understanding the viral transmission processes in insects.
While the specifics of this study were explored in fruit flies, the findings are applicable across a wide range of living organisms from yeast to human cells. The co-senior author, Gary Blissard, a professor of virology, elaborated on the implications: "Viruses have adapted to exploit specific cellular pathways for replication and transmission. Clarifying these pathways is essential for addressing viral infections across species."
Published on August 20 in the Journal of Virology, the study titled "Viral and Cellular Determinants of Polarized Trafficking of Viral Envelope Proteins From Insect-specific and Insect-vectored Viruses in Insect Midgut and Salivary Gland Cells" highlights the dual behavior of viruses. While some viruses are fatal to the host insects and replicate within them, others—vectored viruses—need to navigate multiple tissues while ensuring they cross into the salivary glands to exit and infect a new host effectively.
Viruses utilize spike proteins to access host cells by fitting into specific receptors. In this study, researchers examined two different spike proteins: GP64, derived from a virus that infects only insect hosts, and VSV G, associated with a vectored virus. By engineering fruit flies to express these proteins, the team could monitor their movements within the tissue.
An intriguing aspect of their findings reveals that when viruses infiltrate the insect body, they traverse through the basal membrane of midgut epithelial cells. However, upon reaching the salivary glands, the process inverts; the virus must navigate out through the apical membrane, a movement that employs different cellular mechanisms that remain partially understood.
The experiments revealed that while both spike proteins traveled to the basal side of the midgut cells, they displayed distinct behaviors in the salivary glands. GP64 was restricted to the basal side, indicating its role in insect infection. In contrast, VSV G demonstrated the ability to move apically, suggesting a pathway for the virus to exit the insect and potentially infect a new host.
The implications of this research are profound. Buchon suggests that future advancements may allow scientists to design insects that disrupt viral transmission, trapping viruses in body cavities or salivary glands and preventing their spread.
With the participation of emerging scientists like co-first authors Jeffrey Hodgson and Robin Chen, the research was facilitated by funding from the National Science Foundation and the National Institutes of Health, marking a significant step forward in our understanding of how infections propagate in our ever-connected environment.
Stay tuned as we continue to unfold the mysteries of viral transmission across the animal kingdom, and be prepared—this could lead to a game-changer in how we approach global health challenges!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)