Is Stalled Amyloid Protein Production the Hidden Trigger Behind Alzheimer's Disease? New Study Reveals Shocking Findings!
2025-01-21
Author: Daniel
Overview of the Groundbreaking Study
A groundbreaking study published in eLife has unveiled that the production of amyloid proteins in the brain may be at the core of Alzheimer's disease, throwing new light on years of research focused on amyloid beta (Aβ) accumulation. This research challenges long-standing beliefs about the mechanisms driving this devastating neurodegenerative disorder.
Challenging the Amyloid Cascade Hypothesis
For decades, scientists have been entrenched in the amyloid cascade hypothesis, which suggests that the build-up of Aβ proteins instigates a series of events that eventually leads to brain degeneration and dementia. However, the recent work of a team at the University of Kansas underscores a crucial development: stalled protein processing might be more critical than previously thought.
Insights from the Lead Researcher
Leading the study, Parnian Arafi, a medicinal chemistry research assistant, expressed the urgency of this new direction. "Despite numerous breakthroughs in understanding mutations linked to Aβ aggregation, there’s still a significant gap in understanding how neurotoxic Aβ proteins assemble," Arafi stated. Given that many clinical trials targeting Aβ have shown only limited success, the research calls for a reevaluation of this protein as the primary driver of Alzheimer's pathology.
The Role of Proteolysis
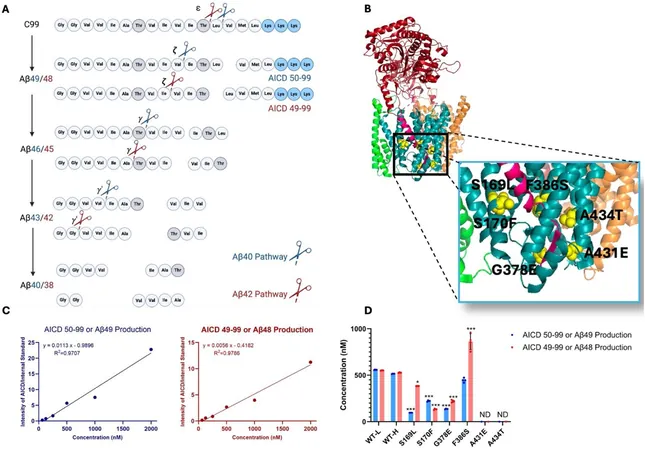
The focus is now shifting to a critical process known as proteolysis, where amyloid precursor protein (APP) is cleaved by an enzyme known as gamma-secretase (γ-secretase). Past studies from the same team have already established that certain mutations in the presenilin-1 (PSEN1) gene associated with familial Alzheimer's disease (FAD) hinder γ-secretase from effectively trimming APP. This dysregulation leads to the accumulation of long, unprocessed forms of the protein, ultimately resulting in cellular dysfunction and neurodegeneration, even in the absence of detectable Aβ.
Examining Mutations and Their Effects
In this pivotal research, the investigators examined a total of six mutations linked with early-onset familial Alzheimer's, which affects individuals as young as 27 years old. They developed and analyzed mutant γ-secretase proteins and measured the unfolding effects on APP processing using cutting-edge mass spectrometry techniques. Their findings revealed that every mutation tested caused notable deficiencies in how APP was processed, with some mutations impacting the production of intermediary proteins to a greater extent than others.
Stability of Enzyme-Substrate Complexes
A fascinating aspect of their analysis involved measuring the stability of enzyme-substrate complexes. By employing fluorescently labeled antibodies, the team was able to determine how mutations affected the binding between γ-secretase and APP, discovering that all mutant forms led to enhanced stability of these complexes—suggesting a standstill in the proteolytic process.
Implications of the Findings
Arafi commented on the implications of these findings: "This research supports our ‘stalled complex’ hypothesis, suggesting these enzyme-substrate complexes could trigger neurodegeneration independent of amyloid beta production." The implications of this notion could lead researchers to explore therapeutic avenues beyond targeting amyloid proteins.
Future of Alzheimer's Research
As the quest to unlock the mysteries of Alzheimer’s disease continues, senior author Michael Wolfe pointed to the promising prospect that if γ-secretase activators can mitigate stalled proteolysis, a new class of treatments may emerge. He emphasized, "Recognizing the role of processes beyond amyloid beta-protein could revolutionize the way we approach Alzheimer’s therapies, particularly for those with genetic predispositions."
Conclusion
The research paves the way for innovative strategies in combatting Alzheimer’s, spotlighting the complexities of neurodegenerative diseases. If this trajectory is further validated, it could lead to breakthrough therapies that not only refine our understanding of Alzheimer’s but also provide hope for millions in search of effective treatment options.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)