How Selection Strength Influences Amino Acid Frequency Evolution Across Species
2025-01-06
Author: Li
In the fascinating world of evolutionary biology, the nearly neutral theory posits that species with larger effective population sizes (Ne) have a heightened ability to eliminate slightly harmful mutations. This theory serves as the foundation for a recent comparative study examining the effects of Ne on amino acid frequency evolution in vertebrates.
Researchers explored the evolutionary dynamics of high-Ne groups, such as rodents and lagomorphs, compared to their low-Ne counterparts, including primates and colugos. To uncover the subtle selective pressures influencing amino acid frequencies, the study employed three complementary methods, providing a comprehensive analysis of evolutionary trends.
The first method involved fitting non-stationary substitution models of amino acid flux through maximum likelihood estimation. This approach helped researchers identify the differences in amino acid substitution rates between high-Ne rodents and lagomorphs versus the low-Ne primates and colugos.
Next, the team expanded their comparison, correlating amino acid frequencies across a broader range of vertebrates to illuminate the relationship between Ne and substitution patterns. This second analysis revealed intriguing trends, linking amino acid selection more clearly to the population dynamics.
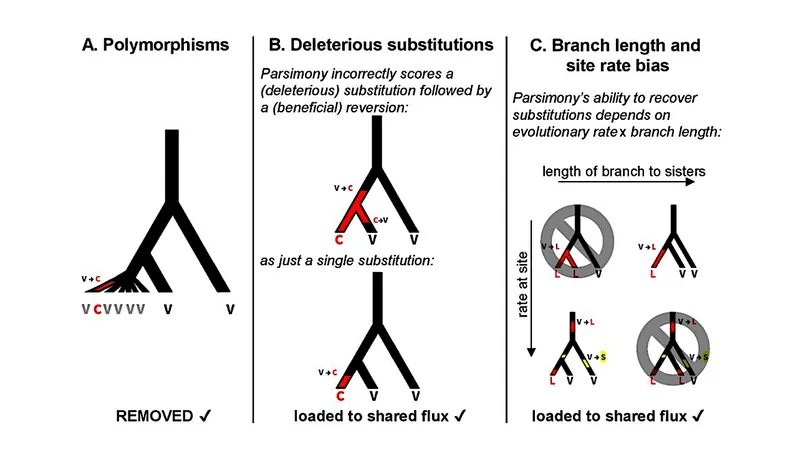
The third approach included a detailed examination of amino acid flux specifically in humans, chimpanzees, mice, and rats. By employing a parsimony-based scoring system, researchers not only identified which amino acids were favored under stronger selection but also compared these findings to historical data.
All three methods converged on the conclusion that specific amino acids were favored in environments with more effective selection. Smaller amino acids that are less energetically expensive to synthesize often showed a preference – particularly those that promote intrinsic structural disorder, a significant feature of protein functionality and stability.
Interestingly, bias from parsimony in historical analyses led to misinterpretations regarding structural disorder, likely due to the impact of slightly deleterious substitutions. Within pairs of highly exchangeable amino acids, such as arginine and lysine, and valine and isoleucine, preferences indicated a tendency towards configurations that allow for a marginally higher free energy of folding – an important aspect for thermophilic organisms compared to their mesophilic relatives.
These findings not only highlight the complex interplay between mutation, selection, and genetic drift but also affirm the nearly neutral theory's relevance in understanding protein evolution. The implications of this research extend to our understanding of how evolutionary pressures shape the very building blocks of life, potentially offering insights into future studies in fields like astrobiology, where the principles of evolution could inform the search for life beyond Earth.
Stay tuned for more groundbreaking discoveries in evolutionary biology that promise to change our understanding of life's intricacies!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)