Groundbreaking Study Unveils Secrets of Ancient Protoribosome: Key to Understanding Life's Beginnings!
2024-10-01
Scientists have made a remarkable discovery that shines a light on the protoribosome—an ancient molecular fossil that may hold the key to understanding how life began on Earth. This exciting research reveals not only the structure of the protoribosome but also its pivotal role in the early stages of life’s evolution.
A collaborative team from prestigious institutions including Charles University and the University of Chemistry and Technology in Prague, University of Milano, and the Institute of Science Tokyo has conducted an extensive study that examines how ancient peptide fragments—resembling the simplest forms of ribosomal proteins—contribute to the stability of protoribosomal RNA. Published in the journal *Nucleic Acids Research*, this study could revolutionize our grasp of ribosomal evolution and its relationship to the origin of life itself.
Life as we know it is built on intricate interactions between nucleic acids, proteins, and lipids. “These molecular interactions began over 4 billion years ago, laying the groundwork for what would eventually emerge as life,” explains Dr. Klára Hlouchová of Charles University, a key figure in the research.
Ribosomes, often regarded as the ancient machinery of life, are found in every living cell, producing proteins essential for countless biological functions. Because of their presence across all forms of life and their high evolutionary conservation, ribosomes are invaluable to evolutionary biologists seeking to understand our biological roots.
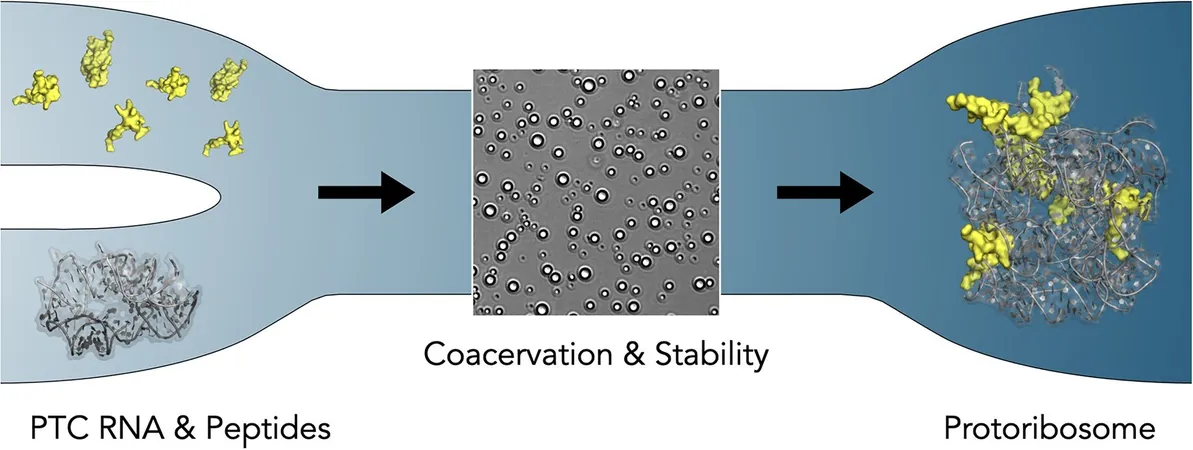
At the heart of the protoribosome lies the peptidyl transferase center (PTC), responsible for forming peptide bonds—a fundamental process for protein synthesis. Fascinatingly, earlier research suggested that RNA alone could be functional in some aspects of this process. However, the study reveals that the "tails" of certain ribosomal proteins (rPeptides) are crucial for maintaining the integrity of the PTC and serve as remnants of ancient peptides that probably interacted with the protoribosome before the evolution of the modern RNA-protein complex.
Through in-depth analysis, the scientists found that rPeptides play a significant role in stabilizing the protoribosome by facilitating compartmentalization. The research focused on two evolutionary stages of protoribosomal RNA: the 617-nucleotide and the 136-nucleotide constructs.
The smaller construct demonstrates greater structural flexibility and interacts with rPeptides with lower specificity, creating liquid-like droplets that help shield RNA from degradation. Conversely, the larger construct is structurally more defined and demonstrates a more intimate interaction with the rPeptides; however, it shows less coacervation compared to its smaller counterpart.
These findings posit that the initial interactions between rPeptides and RNA not only prevented RNA degradation but were also crucial in establishing the primitive compartments necessary for early life's survival. This came long before the emergence of the complex RNA-protein interactions we recognize as foundational to modern ribosomes.
Dr. Hlouchová summarizes the impact of these findings, stating, “Our research suggests that peptides are integral to the condensation and stabilization of the protoribosome, shedding light on how early life processes could have been compartmentalized in a prebiotic world.”
Co-lead researcher Prof. Giuliano Zanchetta further emphasizes the importance of this discovery, noting, “The formation of these concentrated droplets is not random; it depends subtly on the RNA's sequence and structure, highlighting a specific mechanism tailored for ribosomal particles.”
This research not only uncovers vital information about our evolutionary history but also offers profound insights into the biophysical processes that may have protected the building blocks of life during Earth’s primordial era. As scientists continue to break down the barriers of our origins, this latest study brings us one step closer to unraveling the complex tapestry of life's beginnings.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)