Groundbreaking Study Reveals How Overactive Nervous System Fuels Insulin Resistance in Obesity!
2024-11-02
Author: Rajesh
Groundbreaking Study Reveals How Overactive Nervous System Fuels Insulin Resistance in Obesity!
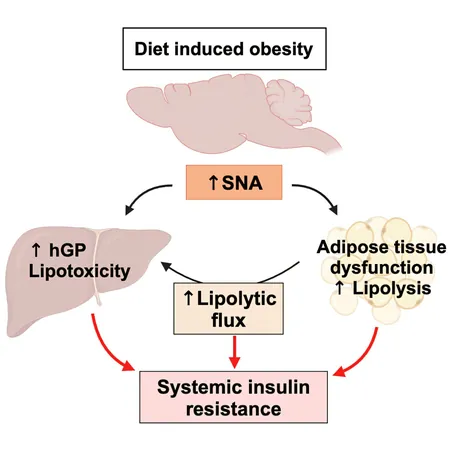
A remarkable new study from Rutgers Robert Wood Johnson Medical School and its partners reveals a shocking connection between overnutrition, the sympathetic nervous system (SNS), and insulin resistance—issues that ultimately lead to type 2 diabetes and metabolic disorders. This groundbreaking research is shifting the conversation on how obesity affects our bodies, uncovering the significant implications for prevention and treatment of diabetes.
Traditionally, the prevailing understanding of insulin resistance centered around impaired cellular insulin signaling. However, researchers now suggest that this isn't the full story. While abnormal insulin signaling has been a recognized factor, it does not always correlate with decreased insulin action. This indicates additional contributors to the development of insulin resistance, pointing to the mysterious role of SNS activity—a topic that has sparked debate in the scientific community.
Overnutrition has previously been known to spike plasma norepinephrine (NE) levels, a clear marker of SNS overactivation. Interestingly, while some studies indicate increased SNS activity in obesity, others have shown conflicting results, suggesting reduced response to adrenergic signals. This paradoxical observation may stem from chronic SNS overactivation, leading to what researchers term "catecholamine resistance." Simply put, even when levels of norepinephrine are high, the body's response may weaken over time.
In a study featured in the prestigious journal Cell Metabolism, the research team explored these ambiguities using a specialized mouse model that selectively removed the gene responsible for producing tyrosine hydroxylase (a key enzyme in catecholamine production) in peripheral tissues. This groundbreaking technique enabled the investigation of the postulated effects of SNS activity while maintaining normal brain functions.
The findings were striking: when wild-type mice were subjected to a high-fat diet (HFD) for just a couple of weeks, they experienced significant weight gain, impaired glucose tolerance, and a marked increase in blood sugar levels, despite elevated insulin levels indicating that cellular signaling pathways were still intact. In contrast, mice genetically modified to exhibit reduced SNS activity displayed remarkable resilience, maintaining glucose tolerance and stable blood sugar levels even when consuming a high-fat diet.
As the study progressed to long-term overnutrition—spanning 12 weeks—the differences became undeniable. Wild-type mice displayed clear signs of catecholamine resistance coupled with increased levels of NE, epinephrine, and glucagon, while the specially modified mice (THΔper) showed lower levels of these hormones. This meant that despite equivalent weight gain, THΔper mice avoided many of the metabolic dysfunctions typically triggered by prolonged HFD exposure.
The implications are profound: the researchers propose this new perspective on the role of the SNS in driving insulin resistance could potentially unlock new avenues for treating and preventing type 2 diabetes.
As we stand on the brink of this research revelation, it is crucial to delve further into how these findings might translate to human health. Could reducing SNS activity help manage obesity-related complications? It's a question that supports the need for ongoing research in this exciting and ever-evolving field. Stay tuned for more insights as science continues to unravel the mysteries of our metabolism!
This study not only challenges conventional wisdom but may redefine our approach to public health strategies targeting obesity and diabetes. What else might we discover about the intricate dance of hormones, diet, and nervous system activity? Only time—and further research—will tell.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)