Groundbreaking Study Reveals How Cancer-Driving Enzyme Could Be Targeted for New Treatments
2025-03-25
Author: Wei Ling
Groundbreaking Study on CDK7 Enzyme
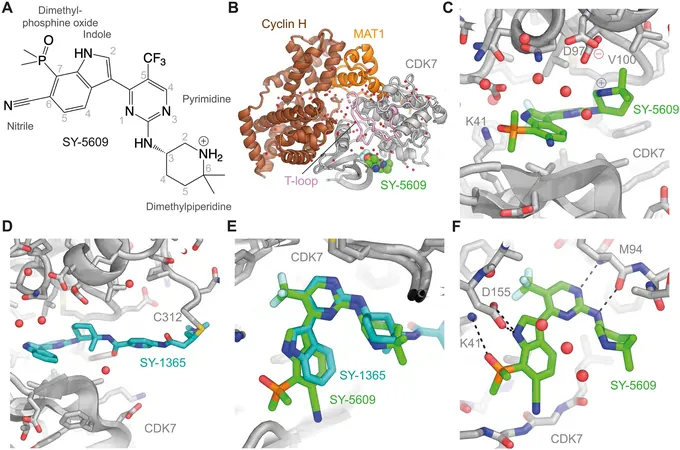
In a remarkable breakthrough, researchers at CU Boulder have unveiled key insights into the functioning of an enzyme called CDK7, which plays a crucial role in cell proliferation. When this vital process spirals out of control, it can lead to the development of cancerous growths.
Published in the prestigious journal *Science Advances*, this innovative research opens the door to novel cancer therapies. The study indicates that experimental drugs designed to inhibit CDK7 can rapidly halt gene expression pathways that are responsible for cell growth across various tissue types.
Significance of the Discovery
Dylan Taatjes, a leading biochemist and senior author of the study, emphasized the significance of this discovery: “This work addresses a long-standing mystery surrounding CDK7, a central player in the regulation of the cell cycle and proliferation. Understanding its role not only enhances our grasp of biological development but also has vast therapeutic implications.”
The Role of CDK7 in Cancer
For years, cancer researchers and pharmaceutical companies have recognized CDK7 as a "master regulator" of cell division. It activates other enzymes, called kinases, that signal cells to divide. Additionally, it regulates crucial transcription factors that dictate how genes are expressed—historically, a process fraught with uncertainties.
While essential for normal growth, CDK7 is often exploited by certain cancers, notably the aggressive and hard-to-treat "triple negative" breast cancers that make up a significant portion of breast cancer diagnoses. Recent efforts have yielded a variety of CDK7 inhibitors in clinical trials; however, their efficacy has been compromised by serious side effects and incomplete tumor eradication.
In-Depth Analysis of CDK7 Mechanisms
The research team, which included Taatjes, Professor Robin Dowell, and graduate student Taylor Jones, undertook an in-depth analysis of CDK7's mechanisms using advanced computational methods. By applying a CDK7 inhibitor from Syros Pharmaceuticals to cancerous human cells, they monitored the immediate effects, revealing that within just 30 minutes, a set of core transcription factors crucial for cell proliferation was universally inhibited.
Universal Mechanism of CDK7
This finding is monumental—it highlights a universal mechanism through which CDK7 governs human cell growth, as Taatjes explained: “As soon as you inhibit CDK7, the transcription factors that drive proliferation are switched off, effectively halting cancer growth.”
Targeting RB1 in Cancer Treatment
Moreover, the team identified the retinoblastoma protein 1 (RB1) as a key player in this process. This protein, a well-known tumor suppressor, typically gets disrupted in cancer. The research suggests that targeting CDK7 could also impact RB1's functionality, potentially leading to new treatment options aimed at this critical tumor suppressor.
Gradual Restraint of Cell Division Enzymes
In addition to swiftly shutting off the core transcription factors, the CDK7 inhibitor also gradually restrains other related enzymes that prompt cell division—though this happens at a slower pace and operates independently of the transcription facts involved.
Implications for Cancer Therapies
The implications of these findings are profound. The potential to craft new therapies that selectively inhibit CDK7’s harmful functions—without entirely shutting down its beneficial roles—could revolutionize cancer treatment. As Taatjes put it, “Rather than using a broad approach that disrupts all CDK7 activities, we could selectively target its actions that contribute to tumor proliferation, minimizing damage to normal cells.”
This could lead to the development of more precise cancer therapies that effectively attack tumors while preserving healthy tissue—ushering in a new era of targeted cancer treatment. The future of cancer care may soon witness a paradigm shift thanks to these insights, transforming how we approach one of humanity's greatest health challenges.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)