Groundbreaking Study Reveals Erenumab as a Game-Changer for Chronic Migraine Sufferers!
2024-09-18
Introduction
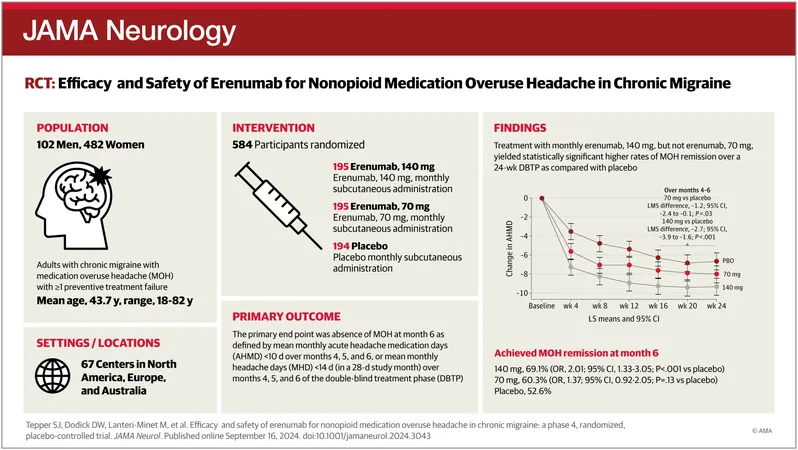
In an exciting breakthrough for chronic migraine (CM) patients, recent research has unveiled that monthly injections of erenumab (140 mg) are not only safe but also highly effective in assisting individuals with medication overuse headache (MOH) stemming from nonopioid use. The findings, published on September 16 in the esteemed JAMA Neurology, could reshape treatment approaches for countless individuals grappling with this debilitating condition.
Study Overview
Led by Dr. Stewart J. Tepper from the New England Institute for Neurology and Headache, the study examined 584 participants who were randomly assigned to receive either erenumab at doses of 70 mg or 140 mg, or a placebo, with injections administered once a month over a span of 24 weeks. The results were nothing short of remarkable.
Efficacy Results
At the six-month mark, a staggering 69.1% of those receiving the higher 140 mg dose of erenumab achieved MOH remission, compared to just 52.6% from the placebo group. Participants on the 70 mg dose showed a remission rate of 60.3%. This indicates that those treated with erenumab experienced a significant enhancement in their quality of life and a reduction in the frequency of debilitating headaches.
Medication Use
Moreover, participants in the 140 mg group reported an astounding average decrease of 9.4 days in their use of acute headache medications each month. In comparison, those on the 70 mg dose saw an 7.8-day reduction, showcasing erenumab’s potent impact on managing headache frequency and related medication use.
Long-term Remission Rates
Importantly, the benefits of erenumab were not only evident at the initial treatment phase; 61.3% of participants in the 140 mg group maintained their MOH remission throughout the treatment period. Even in the 70 mg group, 49.5% sustained remission, while only 37.6% of participants receiving placebo experienced similar success.
Safety Profile
In terms of safety, the incidence of adverse effects in the combined groups (66.8%) was consistent with earlier studies. The most prevalent side effects included constipation (15.2%) and COVID-19 (13.9%), underscoring the need for ongoing monitoring in patients receiving treatment.
Conclusion
This pivotal study stands out as the first controlled trial offering Class I evidence, as classified by the American Academy of Neurology, of a migraine preventive treatment's beneficial effects among those suffering from chronic migraine with nonopioid medication overuse. Experts believe this research could transform treatment paradigms for millions suffering from chronic migraines, steering them toward effective solutions that were previously limited.
As such, healthcare providers may soon need to reconsider the role of erenumab in their migraine prevention strategies, potentially offering new hope in the fight against this often-misunderstood condition. Stay tuned for more updates on this promising development in migraine treatments!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)