Groundbreaking Study on Malaria Treatment: Does Sulfadoxine-Pyrimethamine Stand the Test Against Resistant Strains?
2024-09-26
In an innovative approach to combating malaria in Cameroon, researchers are embarking on a pivotal three-arm, parallel, double-blinded, placebo-controlled, randomized trial known as the Parasite Clearance and Protection from Plasmodium falciparum Infection (PCPI) study. The main goal? To understand how different strains of malaria parasites respond to sulfadoxine-pyrimethamine (SP) treatment, particularly in children aged 3-5 who show no symptoms.
Study Overview
The PCPI study aims to enroll approximately 900 asymptomatic children in a high malaria transmission area within the Malantouen health district, where the prevalence among children under five years can soar to around 40% during the rainy season from April to November. This is crucial timing, as the rainy season exacerbates malaria transmission.
Who's Involved?
Children aged 3 to 5 will be recruited based on specific eligibility criteria. Those showing acute illness or symptoms of malaria will be excluded to ensure the accuracy of results. Parents will play a vital role, as their consent is mandatory for the child’s participation.
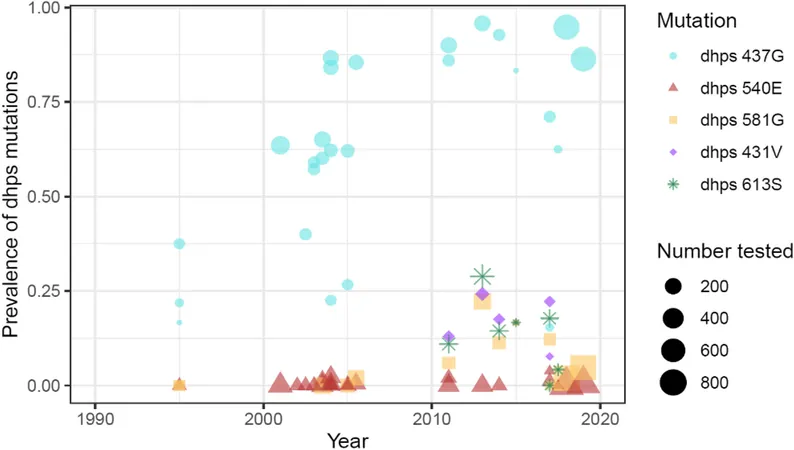
Genotype Focus
The study zeroes in on the dhps 431V mutation, known to confer resistance to SP. Previous estimates indicated that this mutation occurs with a frequency of roughly 27.8% in the Ngounso area, akin to findings from the Northwest region. Researchers will track these genetic profiles to better understand the treatment's efficacy.
Trial Design
Participants will be randomly assigned to one of three groups: one receiving SP alone, another receiving SP with amodiaquine (AQ), and a placebo group receiving only placebos. The randomization ensures a robust comparison of the treatments’ effectiveness over the course of the study.
Comprehensive Follow-Up
Following the treatment protocol, the children will be monitored over a 71-day period through regular follow-up visits. This will include blood tests to confirm whether they are still malaria-free, tracking any symptoms, and managing any adverse events. The rigorous monitoring reflects the researchers' commitment to participant safety.
Data Collection and Analysis
Utilizing REDCap, a secure data capture system, the study will meticulously collect and protect participant information while also leveraging advanced statistical models to predict outcomes. Among the critical objectives is the determination of how effectively the treatments can protect against new infections and clear existing malaria parasites from the body.
Ethical Assurance
The trial adheres to rigorous ethical standards and has received approvals from multiple ethics committees both domestically and internationally, highlighting its commitment to upholding the highest research integrity.
The Impact of Findings
If successful, this study could reshape malaria treatment protocols, especially in regions facing rising resistance to standard therapies. The implications extend beyond Cameroon, potentially offering insights into malaria management strategies worldwide.
In conclusion, the PCPI study stands as a beacon of hope in the fight against one of humanity’s oldest diseases—a battle that entwines science, ethics, and community engagement. The world watches closely as results could open new doors to safeguarding millions from malaria.




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)