Groundbreaking Student-Developed Model Paves the Way for Next-Gen Renewable Fuels!
2024-10-31
Author: Nur
Groundbreaking Student-Developed Model Paves the Way for Next-Gen Renewable Fuels!
In a remarkable achievement, a team of bachelor students from TU Delft has successfully created an innovative model that predicts the molecular properties of alkanes, crucial for advancing renewable fuel technologies. This project was part of their minor in Computational Science and Engineering and has gained attention by being published in The Journal of Physical Chemistry B.
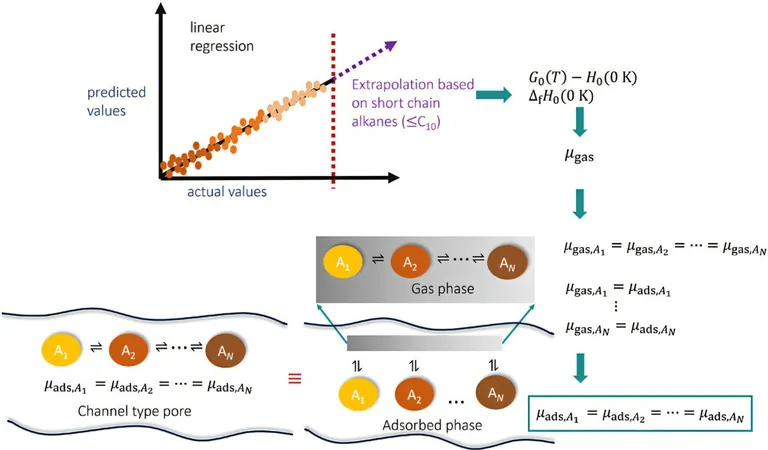
The transition to renewable energy sources is no longer a distant goal; it is rapidly becoming our reality. However, the journey towards efficient production techniques remains fraught with challenges. As noted by Professor Thijs Vlugt, an expert in Engineering Thermodynamics, "The demand for sustainable aviation fuel is rising, and shorter alkanes, which are hydrocarbon molecules, are deemed highly effective due to their advantageous molecular characteristics." A key part of manufacturing these fuels involves breaking down longer alkanes into shorter ones using zeolites, materials that feature nanopores, but the exact chemical processes at play are still not fully understood.
Over the course of six months, students Josh Sleijfer, Jeroen op de Beek, Stach van der Zeeuw, and Daniil Zorzos immersed themselves in this complex issue while juggling their regular academic commitments. “Our primary objective was clear: to develop a model that could provide a more accurate prediction of longer alkanes a topic that has seen limited exploration,” states Sleijfer.
What sets their model apart is its comprehensive approach. Unlike traditional models that concentrate only on carbon atoms and their direct neighbors, this new method delves deeper by considering "neighbors of the neighbors." This broader perspective significantly enhances the accuracy of predictions, leading to better understanding and innovation in renewable fuel production.
“The potential structures for alkanes are immense. For instance, a molecule with 20 carbon atoms can present a jaw-dropping 250,000 variations, making measuring every possible structure impractical,” explains Professor Vlugt. As a result, a dependable computer model like this is invaluable for researchers seeking to optimize processes in zeolites for producing renewable fuels.
Fresh perspectives often yield groundbreaking solutions, and the students' varied academic backgrounds helped bring unique insights to the project. Sleijfer, who has dual degrees in Applied Mathematics and Applied Physics, admits, “Initially, the chemistry aspect was challenging. However, once I grasped the fundamentals, everything clicked.”
The students’ innovative work not only made substantial contributions to Vlugt's research but also culminated in a scientific publication that enhances their academic resumes. Few students can boast of a published paper before beginning their master's programs, a badge of honor that Sleijfer wears proudly. Currently pursuing a joint master's in Computer Simulations for Science and Engineering, he notes, "The experience was highly motivating knowing our work had real-world applications."
Given the success of their model, Vlugt and his team are eyeing potential extensions to the model, inviting future student groups to tackle new challenges. The journey towards making renewable fuels the standard continues, thanks to the innovative contributions of these bright students!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)