Groundbreaking Research Unveils Manganese as a Game-Changer for Lithium-Ion Batteries!
2024-09-26
Author: Arjun
Groundbreaking Research Unveils Manganese as a Game-Changer for Lithium-Ion Batteries!
In a pioneering development that could revolutionize rechargeable lithium-ion batteries, scientists at Lawrence Berkeley National Laboratory (Berkeley Lab) have spotlighted manganese as a revolutionary alternative to traditional battery materials.
With the soaring demand for lithium-ion batteries required for smartphones, electric vehicles, and energy storage systems, the looming supply chain challenges tied to nickel and cobalt have synthesized a call for innovation.
The latest study indicates that manganese, an element abundantly found in the Earth’s crust, could be the cost-effective and safer solution we’ve been waiting for!
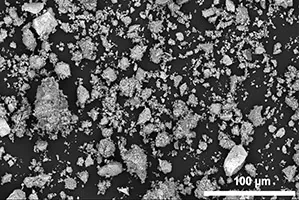
Published in *Nature Nanotechnology* on September 19, the research reveals that manganese can be successfully utilized in disordered rock salt (DRX) cathodes, which is a cutting-edge battery material.
Prior assumptions held that these DRX materials must be ground down into nanoscale particles to excel, a process notoriously energy-intensive.
However, the Berkeley team has discovered that manganese-based cathodes can maintain efficiency even with particles that are 1,000 times larger than previously thought, a revelation that could substantially lower manufacturing expenses!
Han-Ming Hau, a Ph.D. candidate at UC Berkeley and integral part of Berkeley Lab’s Ceder Group, emphasized the significance of this breakthrough: “There are numerous ways to harness power through renewable energy, but the true challenge is effective storage.
Our innovative approach harnesses a material that is both plentiful and economical, needing less energy and time in production compared to current lithium-ion materials—all while delivering equivalent energy storage!”
The research team employed a novel two-day process to fabricate manganese-based cathodes.
This method involves extracting lithium ions from the material and subjecting it to heat of approximately 200°C, a remarkable efficiency compared to traditional techniques that can take more than three weeks!
Advanced electron microscopy provided atomic-level imaging, illustrating that a semi-ordered nanoscale structure capable of enhancing battery performance is achieved during this process.
Moreover, the researchers conducted in-depth X-ray analyses, tracking the material changes during battery cycling, which yielded invaluable insights into its chemical dynamics.
This comprehensive examination of the manganese material is a significant stepping stone toward future advancements in nano-engineering and cathode production.
“This new understanding of the unique nanostructure and the synthesis process needed to boost its electrochemical performance propels us closer to implementing this material in commercial batteries,” Hau stated, stirring excitement within the scientific community.
This groundbreaking research has utilized facilities from various Department of Energy (DOE) labs, such as the Advanced Light Source, Molecular Foundry, and National Synchrotron Light Source II.
Financial backing flowed from the DOE's Office of Energy Efficiency and Renewable Energy and the Office of Science.
As the global shift towards clean energy accelerates, the identification of manganese as an effective cathode material could not only alleviate concerns regarding materials sourcing but also herald a new era of more sustainable energy storage solutions.
Could this be the dawn of a battery revolution? Stay tuned!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)