Groundbreaking Research Unveils Dual Nature of Alpha-Synuclein Inclusions in Neurodegenerative Disorders
2024-09-20
Author: Wei Ling
Introduction
In a significant breakthrough for neurodegenerative disease research, scientists have discovered that not all alpha-synuclein inclusions are harmful—their effects can vary dramatically, according to a recent study published in the September 2024 issue of Neuron. Led by Vikram Khurana at Brigham and Women's Hospital in Boston, the team employed an innovative stem cell technique to explore the inclusionopathies associated with conditions like Parkinson's and Alzheimer's diseases.
Methodology
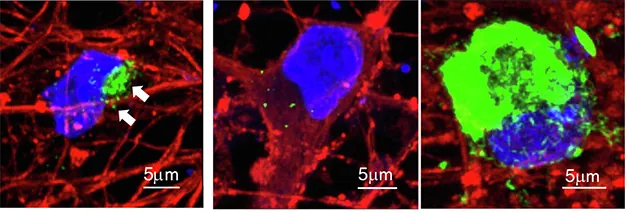
Using induced pluripotent stem cells (iPSCs), the researchers were able to differentiate these cells into neurons that produced inclusions resembling those found in the brains of individuals with synucleinopathies. They identified two major forms of inclusions: one being a lipid-rich, unstable form that accumulated primarily in neuronal cell bodies, and another much more stable form loaded with fibrillar alpha-synuclein aggregates and the autophagy protein p62.
Key Findings
Interestingly, while the unstable inclusions proved toxic to the neurons, the stable, fibrillar aggregates appeared to have neuroprotective properties. This discovery suggests that the composition of alpha-synuclein aggregates is linked to their distinct cellular effects, a finding that could help tailor therapeutic approaches in the future.
Research Models
Khurana's team has established over 60 different cell models of inclusionopathies, which express various forms of alpha-synuclein and tau proteins. These models offer a platform for researchers globally to investigate the nuances of neurodegenerate diseases in relevant human cell types.
Community Response
Prominent figures in the research community, like Mark Cookson from the National Institutes of Health (NIH), have praised this work as 'state of the art.' He highlighted the importance of studying the distinct properties and toxicities of these inclusions, as they could inform the development of therapeutic strategies that selectively target only the harmful aggregates.
Innovative Techniques
The team’s innovative use of high-capacity piggyBac vectors to accelerate the differentiation of iPSCs into neurons has drawn significant attention. By integrating genes related to neurodegeneration into the genome, they could produce neurons that quickly express aggregate-prone proteins, thus providing valuable insights into the cellular mechanisms of diseases.
Types of Aggregates
When examining neurons expressing the A53T-mutated alpha-synuclein, the researchers noted two distinct types of aggregates. Type I inclusions, which were lipid-rich and dynamic, dissolved rapidly under certain treatments, while Type II inclusions, composed of filamentous alpha-synuclein, were stable and varied in their association with the protective protein p62.
Real-world Implications
Intriguingly, these results align with findings from postmortem human brain samples, suggesting that the nature of these inclusions is also reflected in actual disease conditions.
Future Directions
Moving forward, the research team aims to uncover the mechanisms that govern the formation of these different types of inclusions and explore strategies to shift the balance from harmful to protective aggregates. They have already identified a correlation between neurotoxicity and the sequestration of actin-modulating proteins, signifying the complexity of interactions within neurodegenerative pathology.
Conclusion
Khurana's research emphasizes the pressing need to refine our understanding of alpha-synuclein's role in neurodegeneration, particularly as pathological loading doesn't always correlate with neuronal damage in human cases. This ongoing investigation opens new avenues for targeted therapies that could mitigate the effects of toxic inclusions while harnessing the potential protective properties of stable aggregates.
Implications for Treatment
As this pioneering work continues to unfold, it promises not only to deepen our understanding of neurodegenerative diseases but also to pave the way for potentially transformative treatment strategies in the fight against these debilitating conditions.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)