Groundbreaking Research Unveils Cancer Drug Avapritinib’s Potential to Combat Aggressive Brain Tumors
2025-03-14
Author: Mei
Overview of Avapritinib and its Research
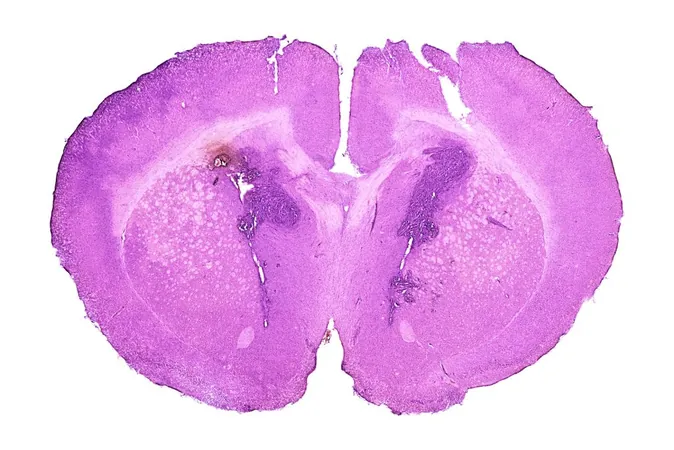
In a remarkable breakthrough, researchers from the University of Michigan, Dana-Farber Cancer Institute, and the Medical University of Vienna are shedding new light on avapritinib, an FDA-approved medication that has shown promising potential in targeting high-grade gliomas (HGG), an aggressive form of brain cancer notoriously difficult to treat. Their findings, published in the prestigious journal *Cancer Cell*, reveal that avapritinib effectively inhibits PDGFRA signaling in brain tumors harboring mutations in the PDGFRA gene, a critical driver of tumor growth.
Promising Efficacy and Mechanism of Action
Dr. Carl Koschmann, a leading researcher and clinical scientific director at the Chad Carr Pediatric Brain Tumor Center at Michigan Medicine, expressed enthusiasm about the drug's efficacy. "We were excited to see that avapritinib essentially shut off PDGFRA signaling in mouse brain tumors," he stated, indicating the potential of this repurposed drug.
Background and Rationale
Originally developed for gastrointestinal stromal tumors and systemic mastocytosis, avapritinib’s unique ability to cross the blood-brain barrier positions it as an attractive option for glioma treatment. Alterations in the PDGFRA gene occur in approximately 15% of pediatric HGG cases, making it a promising therapeutic target. Given the grim prognosis associated with HGG—with many patients surviving less than two years, particularly after recurrence—this research opens new avenues for treatment.
Screening and Findings
To identify effective treatments for HGG, the research team screened various next-generation tyrosine kinase inhibitors (TKIs) targeting PDGFRA, ultimately finding avapritinib to be the most potential candidate. Their lab results demonstrate that HGG models with PDGFRA gain or amplification respond positively to the drug, indicating that this treatment could extend beyond the targeted D842V mutation for which avapritinib was originally designed.
Preclinical Validation
Further validating its capabilities, preclinical studies using animal models showed avapritinib's proficiency in penetrating the blood-brain barrier, a major obstacle in treating brain tumors. Dr. Kallen Schwark, an MD/PhD student involved in the research, noted that the successful delivery of the drug to the brain suggested significant promise.
Initial Clinical Trials and Patient Response
Based on encouraging results from mouse models, researchers proceeded to administer avapritinib to an initial group of eight patients with high-grade gliomas through an expanded access program. The findings were optimistic; three out of seven evaluable patients experienced tumor shrinkage, reinforcing the drug's potential efficacy in human subjects. The researchers highlighted that they gained insights into possible genetic predictors of avapritinib’s effectiveness in HGG patients with PDGFRA copy-number gains, a factor associated with poorer prognoses.
Advantages Over Previous TKIs
What sets avapritinib apart from earlier generations of TKIs is its ability to effectively inhibit PDGFRA within the brain and cross the challenging blood-brain barrier. This distinction elevates its status as a more viable treatment option for specific HGG tumors.
Future Directions and Concerns
Dr. Koschmann remarked, "We have very few examples of drugs entering brain tumors like this and shutting down key oncogenic pathways. These results support ongoing efforts to build on the success of avapritinib and other brain-penetrant small molecule inhibitors." The initial successes have sparked interest in expanding clinical trials, including an ongoing Phase I trial focusing on pediatric solid tumors like HGG.
There is also an ongoing exploration of potential combinations of avapritinib with other therapies to counter drug resistance that can arise in advanced disease stages. Researchers have also raised significant questions regarding the drug's effectiveness on different glioma subtypes. While avapritinib has shown promising results in tumors with PDGFRA amplification, its effectiveness in tumors with other genetic mutations, such as EGFR, remains uncertain. This suggests a pressing need for personalized treatment strategies tailored to the specific genetic profiles of tumors.
Conclusion
Dr. Koschmann emphasized, "We know a single drug is not going to be enough for this disease. The key to making meaningful progress lies in combining various treatment modalities and targeting pathways activated by initial therapies." As this exciting research unfolds, the medical community holds its breath, hoping for a transformative breakthrough that could change the fate of countless patients suffering from this devastating form of cancer.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)