Groundbreaking Progress: Researchers Develop Aluminum-Based Rotaxanes and Polyrotaxanes!
2024-09-19
Introduction
In an exciting development in molecular chemistry, researchers have successfully constructed aluminum molecular ring-based rotaxanes and polyrotaxanes, showcasing fascinating new possibilities in the realm of mechanically interlocked molecules. These rotaxanes, known for their intricate structures of interlocked axles and macrocycles, have become a focal point in the field, promising novel applications in materials science and nanotechnology.
Background on Rotaxanes
Traditionally, a variety of organic macrocycles—such as crown ethers, cyclobis(paraquat-p-phenylene), and cyclodextrins—have paved the way for rotaxane construction. However, the introduction of inorganic metal ions adds a new dimension, enabling scientists to finely tune macrocycles at the molecular level. Until recently, only one type has been fashioned into polymeric rotaxanes using a specific "axle-donor...ring-acceptor" assembly method, posing challenges in achieving a systematic polymeric structure from hybrid macrocycles.
Pioneering Study
A pioneering study published in *Angewandte Chemie International Edition* details a groundbreaking approach from a research team at the Fujian Institute of Research on the Structure of Matter, led by Professors Zhang Jian and Fang Weihui. They have proposed an innovative "ring-donor...axle-acceptor" configuration utilizing Al8 molecular rings, facilitating the stepwise assembly of molecules and polymers through specialized coordination chemistry.
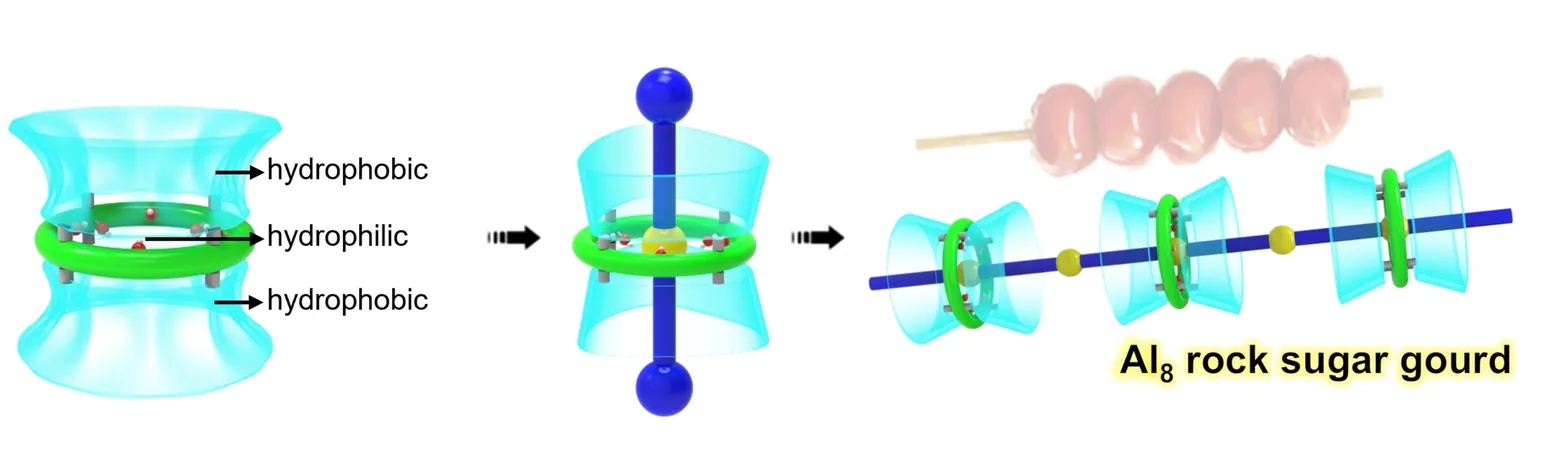
Unique Design of Al8 Macrocycle
The unique design of the Al8 macrocycle includes a tubular cavity featuring one hydrophilic ring (Al8(OH)8) alongside two hydrophobic ports, endowing it with exceptional binding capabilities. The presence of inward-facing hydroxyl (OH) groups creates an ideal environment for the macrocycle to interact with a diverse array of guests—ranging from anions to organic ligands—through its "ring-H/axle-acceptor" binding dynamics.
Construction of Axle Structures
Researchers encapsulated various aromatic compounds within the Al8 structure, including single-site carboxylic acids and dual-site bipyridine compounds. This strategic placement allows for the potential construction of robust axle structures by incorporating metal cations such as Ag+ and Na+, both of which align seamlessly due to their linear coordination geometry.
Formation of [2]-Rotaxanes and Polyrotaxanes
Through meticulous experimentation, the team generated multiple [2]-rotaxanes that consist of inner axles like Ag(bpy)2+ and Na(AQS)2^–. Taking advantage of the uncoordinated nitrogen sites in these complexes, further polymerization led to the formation of a one-dimensional infinite polyrotaxane. In this structure, neighboring [2]-rotaxanes interlink tightly via Ag+ cation interactions, reminiscent of the traditional Chinese treat Tanghulu, where skewers of fruit are coated in sugar.
Nonlinear Optical Properties
These novel host-guest complexes demonstrate remarkable nonlinear optical (NLO) properties, with enhanced absorption responses. Notably, the incorporation of heavy metal cations significantly boosts these characteristics, amplifying the conjugation of organic guests and encouraging polymerization.
Conclusion
Among the various constructs, the Ag(NA)2^– embedded [2]-rotaxane stands out with superior NLO performance, yielding the highest nonlinear absorption coefficient and lowest limiting threshold compared to existing organic molecules and conventional materials. This pioneering research not only heralds a new assembly strategy for rotaxanes and polyrotaxanes but also revolutionizes the understanding of molecular binding dynamics. The introduction of the flexible "ring-donor/axle-acceptor" method marks a substantial advancement in modern molecular chemistry, opening the door to unexplored fields of application—and who knows what groundbreaking innovations might come next!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)