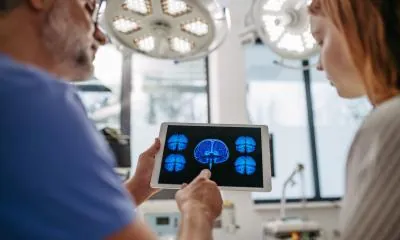
Groundbreaking Non-Invasive Sensor Revolutionizes Intracranial Pressure Monitoring
2025-03-16
Author: John Tan
In a significant medical breakthrough, Brazilian company brain4care has unveiled an innovative sensor capable of non-invasively measuring intracranial pressure (ICP), a critical parameter for patients with neurological conditions. Funded by FAPESP’s Innovative Research in Small Businesses Program (PIPE), this cutting-edge technology offers a game-changing alternative to traditional invasive ICP monitoring methods.
The sensor, which is placed on a patient's head, detects nanometric expansions of the skull with each heartbeat. By analyzing these expansions in real time, the device generates data indicating variations in both volume and pressure inside the skull. An artificial intelligence platform processes this information, assisting physicians in making timely and informed treatment decisions.
Gustavo Frigieri, a key figure behind this technology, highlights the unique approach of brain4care’s system. Unlike conventional methods that prioritize numerical values, this new solution focuses on three critical components of ICP analysis: the numeric value, the trend (whether pressure is increasing or decreasing), and the morphology of the pulse. This method enables early detection of potential changes in a patient’s condition, allowing healthcare providers to intervene before symptoms worsen.
Historically, invasive ICP measurements are reactive—treatment is often initiated only after significant issues arise. In contrast, brain4care offers a proactive solution, providing continuous insights into a patient's ICP status. Frigieri emphasizes, “We aim to empower physicians to act swiftly based on real-time trends rather than waiting for concerning numerical indicators.”
The impact of this non-invasive approach can be profound, especially in critical care scenarios. Prompt detection and intervention can drastically affect patient outcomes, reducing the chances of severe complications or death. The device is particularly valuable in intensive care units (ICUs) where patients are often sedated and unable to display symptoms, allowing physicians to monitor their condition closely.
Having already established credibility through over one hundred published studies, brain4care has been rigorously tested with patients from Brazil, the United States, and Europe. Researchers have successfully estimated ICP values using a machine learning model, achieving an impressive error margin of just 2.6 millimeters of mercury (mmHg) in extensive trials.
Frigieri points out that this non-invasive sensor is versatile, making it applicable beyond the ICU. It can be utilized in clinics, outpatient facilities, and emergency rooms, thus broadening access to essential ICP monitoring. Rapid diagnosis and intervention are critical—especially in cases of head trauma—where delays can significantly impact brain preservation.
Currently, brain4care’s system is operational in over 85 medical institutions across Brazil, from leading hospitals in São Paulo to rural charity facilities. Following its successful domestic launch, the company has begun its international expansion, firmly establishing its presence in the U.S. market.
The technology has secured approval from essential regulatory bodies, including the National Health Surveillance Agency (ANVISA) in Brazil and the Food and Drug Administration (FDA) in the United States. This endorsement positions brain4care as a pioneer in the medical field, opening doors for further research and innovation.
With continued efforts to refine the device and minimize error rates, brain4care is poised to not only enhance patient care but also fundamentally transform the landscape of intracranial pressure monitoring in medicine.
Stay tuned for more developments on this revolutionary technology that could redefine neurosurgery and critical care practices worldwide!





 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)