Groundbreaking Nickel-Boron Complexes: A Catalyst Revolution Awaits!
2025-03-25
Author: Nur
Groundbreaking Nickel-Boron Complexes: A Catalyst Revolution Awaits!
In a fascinating development that could reshape the landscape of catalysis, researchers from the University of Osaka have unveiled a novel chemical bond between nickel(0) and boron, marking a significant advancement in the field of transition metal chemistry. This discovery, recently published in the Journal of the American Chemical Society, opens up exciting new possibilities for the creation of catalysts that are crucial in the synthesis of a variety of essential materials.
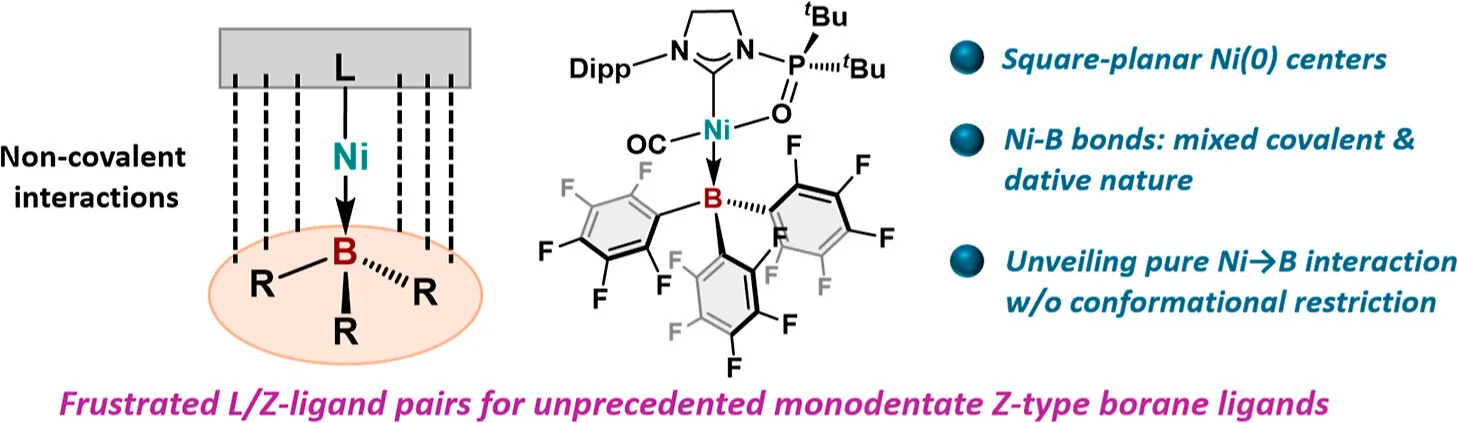
The arrangement of ligands—small molecules that surround transition metal atoms—plays a critical role in determining the reactivity and functionality of these metals. Traditionally, transition metals have been known to form complexes with ligands that include elements from group 13 of the periodic table, such as aluminum and gallium. These ligands, classified as Z-type, are capable of accepting electrons from metal atoms. Until now, however, boron, the smallest group 13 element, had only been observed forming bonds under specific conditions with additional ligands aiding in the process.
In a pioneering study, the Osaka researchers introduced bulky tris(perfluoroaryl)boranes as boron-containing ligands. Remarkably, they succeeded in creating a direct bond between nickel and boron without any constraints imposed by additional ligands. This innovative approach involved both experimental and theoretical techniques, confirming the existence of the nickel-boron bond.
"We found that these boranes can attach to a single site on the nickel atom—an arrangement referred to as monodentate—allowing them to accept electrons directly from nickel," notes lead author Yutaka Mondori. The resulting complex showcases a square-planar geometry, where the nickel atom sits at the center and the ligands are symmetrically positioned, providing access from multiple angles for potential reactants.
Although square-planar arrangements are more typical for nickel in its 2+ oxidation state, this new complex demonstrated an intriguing electronic configuration, characterized by both covalent and dative bonding traits. Additionally, the bulky borane ligands interact with other ligands in the complex, forming what the researchers describe as "frustrated L/Z-ligand pairs,” a concept that could revolutionize the understanding of ligand interactions in catalysis.
"Nickel is a pivotal material used across various catalytic processes," said senior author Yoichi Hoshimoto. "By demonstrating that nickel-boron bonds can be effectively formed with monodentate Z-type ligands, we are unlocking new avenues for refining nickel complexes tailored to achieve specific catalytic outcomes."
This breakthrough not only expands the toolkit available for chemists but also holds tremendous potential for enhancing the efficiency and specificity of catalysts in the production of high-value materials, including advanced polymers and life-saving pharmaceuticals.
Stay tuned as the implications of this discovery unfold, potentially leading to a new era in catalytic science!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)