Groundbreaking Imaging Technique Unveils How Ozone-Damaging Molecule Bromoform Reacts to Light
2024-11-08
Author: Ming
Introduction
In a landmark study, researchers have achieved an unprecedented feat: observing the atomic rearrangement of bromoform in less than a trillionth of a second after exposure to ultraviolet (UV) light. This innovative imaging technique has enabled scientists to visualize a long-anticipated transformation pathway of this molecule known for its detrimental effects on the ozone layer.
The Role of Ultraviolet Rays
Ultraviolet rays from the sun play a significant role in various chemical reactions occurring on Earth, and understanding these ultrafast processes at the atomic level is essential for mitigating their negative impacts on our environment. As senior scientist Oliver Gessner from the Lawrence Berkeley National Laboratory explained, "It's crucial to grasp how electrons and atoms interact to drive certain chemical reactions. Bromoform provides a valuable model for probing these interactions."
Bromoform and Ozone Depletion
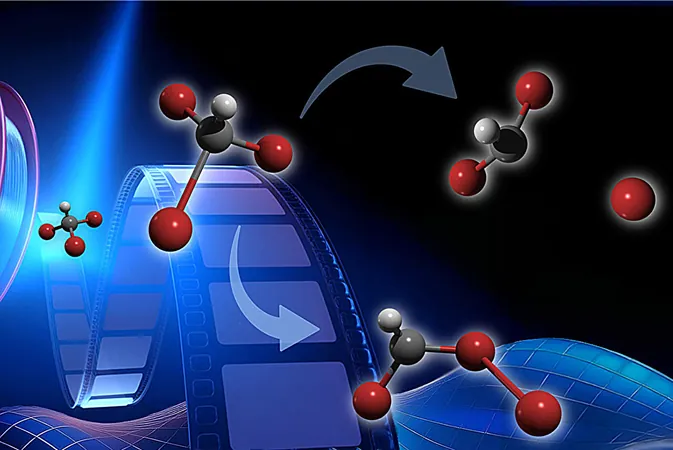
Bromoform has been a subject of intense global study within the field of UV photochemistry for decades. This naturally occurring compound is primarily released by phytoplankton and seaweeds from oceans, contributing to ozone depletion in the atmosphere. Theoretical models suggest that when exposed to UV radiation, bromoform undergoes two significant processes: dissociation, where one bromine atom detaches, and isomerization, where the molecule's structure rearranges into a different isomeric form.
Challenges in Research
Gessner pointed out that while some researchers claim to have detected signs of this isomerization, the fleeting nature of the reactions made it difficult to confirm. Additionally, varying theoretical models have led to substantial discrepancies in predictions regarding the pathways taken by bromoform under UV light.
Experimental Setup and Findings
In their recent publication in the Journal of the American Chemical Society, Gessner and his team presented an experimental setup that not only verified the formation of the isomer but also quantified the proportions of bromoform molecules undergoing dissociation vs. isomerization.
The experiment involved exciting bromoform gas molecules with a burst of UV light at a wavelength of 267 nanometers, followed by imaging these excited molecules with ultrashort electron pulses at the SLAC National Accelerator Laboratory. This advanced relativistic ultrafast electron diffraction instrument allowed them to gather data faster than the rapid chemical processes taking place.
"We needed to outpace the molecules' decisions, which occur within just a few hundred femtoseconds," Gessner noted.
Results and Implications
Analysis of the electron images revealed that approximately 60% of bromoform molecules underwent isomerization within the initial 200 femtoseconds and remained stable throughout the 1.1-picosecond-long study. "It was thrilling to observe the exact isomer configuration that researchers had predicted," Gessner expressed. Meanwhile, the remaining 40% experienced direct dissociation.
This groundbreaking work represents a pivotal advancement in understanding the photochemistry of bromoform and the broader category of UV-induced reactions. "Understanding these chemical pathways is crucial, as the sequence of reactions influences the end products formed," stated Gessner.
Conclusion
This study not only provides a benchmark measurement for a long-open debate regarding isomer formation rates but also holds the potential to refine existing theories governing such reactions. The methodology developed through this research illustrates the power of ultrafast imaging techniques in revealing the rapid dynamics of molecular transformations and could pave the way for future research into complex chemical interactions.
Stay tuned as we uncover more revolutionary breakthroughs in the world of chemistry that could reshape our understanding of atmospheric science and environmental protection!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)