Groundbreaking Discovery: Scientists Unveil How Electrolyte Composition Influences CO₂ Reduction Selectivity with Innovative MOF Catalyst
2024-09-25
Author: Sarah
Introduction
In a significant advancement towards sustainable energy solutions, researchers are shedding light on the electrochemical reduction of CO₂, which has emerged as a promising pathway for utilizing greenhouse gases.
While extensive research has traditionally centered on catalyst design, a recent study emphasizes the critical role of electrolyte composition in controlling product selectivity during CO₂ reduction reactions.
Research Team and Publication
A team of scientists, including Prof. Cao Rong and Prof. Zhang Teng from the Fujian Institute of Research on the Structure of Matter at the Chinese Academy of Sciences, has published their findings in Angewandte Chemie International Edition.
The FICN-8 Catalyst
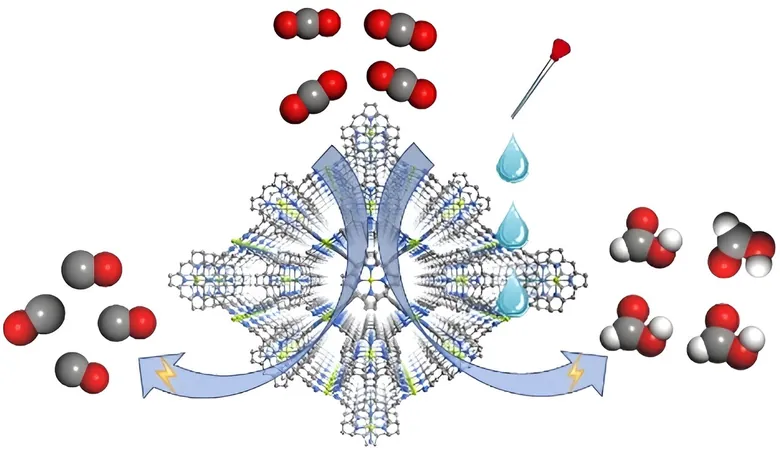
They have developed a cutting-edge metal-organic framework (MOF) catalyst, designated FICN-8, which showcases remarkable selectivity for CO₂ reduction products depending on the electrolytic environment.
FICN-8 is constructed using Cu(porphyrin)-derived ligands and Cu(pyrazolate) building units, resulting in a three-dimensional porous structure.
This design not only maximizes the accessibility of metalloporphyrin catalytic sites but also allows for versatile tuning of solvent and electrolyte compositions—a breakthrough that could facilitate the optimized generation of valuable products.
Electrochemical Testing
Electrochemical testing of FICN-8 revealed its extraordinary activity in CO₂ reduction within a tetrabutylammonium hexafluorophosphate (TBAPF6)/acetonitrile (MeCN) electrolyte.
Astoundingly, the catalyst achieved a remarkable CO selectivity rate of up to 95%.
Effect of Water and TFE
However, a pivotal shift occurred when water or trifluoroethanol (TFE) was introduced into the electrolyte, prompting a change in the main CO₂ reduction product from carbon monoxide (CO) to formic acid.
With the right concentrations—2.65 mol/L of water or 0.55 mol/L of TFE—the researchers recorded an impressive Faradaic efficiency for formic acid production of 48%.
Mechanisms of Selectivity
To further delve into the mechanisms behind the observed selectivity switch, the researchers conducted various experiments including kinetic isotope effect (KIE) measurements.
The findings revealed critical differences in reaction pathways: a near-identity KIE value for CO production, contrasting sharply with a KIE value of 3.7±0.7 for formic acid production.
This strongly suggests that protons play a direct role in the formation of formic acid.
Theoretical Calculations
Moreover, theoretical calculations have pinpointed reductive adsorption of hydride (H) at the porphyrin nitrogen site as a vital step for forming HCOOH.
In contrast, the production of CO operates via an independent route, unaffected by proton concentrations.
Conclusions and Implications
This monumental discovery not only highlights the importance of electrolyte composition in catalysis but also opens paths for further research into optimizing CO₂ reduction methods—potentially revolutionizing our approach to carbon capture and conversion technologies.
With increasing pressure to mitigate climate change effects, this breakthrough could pave the way for developing more efficient systems to turn carbon emissions into usable resources.
The potential impact on renewable energy and sustainability is immense, inviting future investigations into how these findings can be applied in real-world scenarios.
Stay tuned for more updates on novel advancements in the field of electrochemistry and sustainability!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)