Groundbreaking Discovery Reveals Secret Life of Catalysts: What You Never Knew About Vinyl Acetate Production!
2025-04-03
Author: Wei
Introduction
In the realm of chemistry, catalysis plays a pivotal role in speeding up chemical reactions that produce many of the substances we use daily. However, a profound mystery shrouded this process for years: how exactly do these catalysts function? Thanks to a novel study from Massachusetts Institute of Technology (MIT), we now have fresh insights that could transform our understanding.
Key Findings
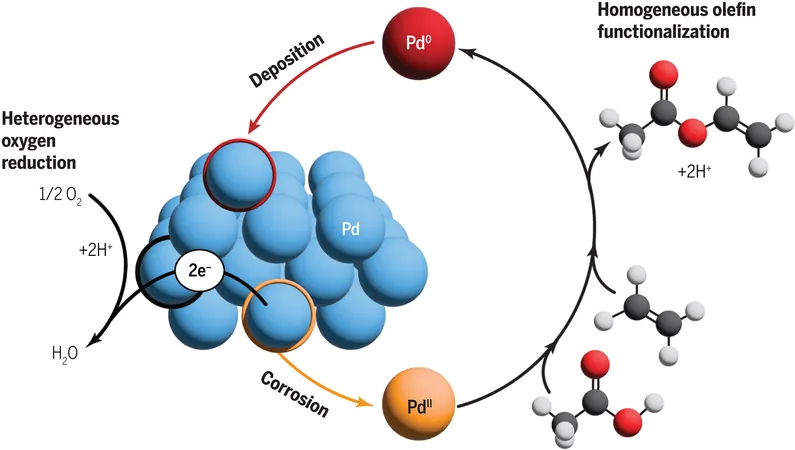
Researchers have uncovered that during the production of vinyl acetate—an essential compound utilized in manufacturing polymers like shoe rubber—a catalyst must oscillate between two distinct forms. This revelation defies the long-held belief that only one form was essential for the reaction.
Published in the esteemed journal Science, the study, led by MIT graduate students Deiaa Harraz and Kunal Lodaya, along with Bryan Tang, Ph.D., and chemistry professor Yogesh Surendranath, depicts a complex dance of catalysts that has significant implications for industrial chemistry.
Catalyst Types
Catalysts can be classified broadly into two categories: homogeneous catalysts, which operate in solution, and heterogeneous catalysts, which are solid materials providing surfaces for reactions. Surendranath emphasized that the previously rigid paradigm kept researchers from exploring the dynamic interactions that can occur between these two types.
"What's groundbreaking about our findings is that both forms of catalysis are inherently linked," Surendranath stated. "In essence, you have these solid metal catalysts converting to molecules and vice versa, creating a continuous cycle that enhances efficiency."
Historical Context
Vinyl acetate synthesis has been an industrial staple since the 1960s, refined largely by trial and error. The discovery that both types of catalysis play critical roles could shift paradigms in how chemists and engineers approach this process. While homogenous catalysis is often the focus for chemists, and surface reactions attract the attention of chemical engineers, only a few researchers have equal expertise in both. This lack of interdisciplinary study has hindered a comprehensive understanding of the catalytic complexities at play.
Research Methodology
To further dissect this interplay, the research team examined the activation process of the reaction constituents: oxygen molecules, acetic acid, and ethylene. They discovered that the optimal catalytic form for one reaction component was not the same for others. The solid catalyst facilitated oxygen activation, while its molecular counterpart engaged directly with ethylene and acetic acid.
Corrosion Analogy
Remarkably, the transition between these two catalyst forms mimics the partial corrosion processes seen in rusting. Surendranath explained that rusting involves the formation of soluble species, an aspect they leveraged by applying corrosion research techniques to their studies. By employing electrochemical methods, they dove deeper into the reaction, revealing that the transformation from solid palladium to soluble palladium ions results from an electrochemical interaction with oxygen.
Conclusion and Future Implications
The researchers postulated that the rate of this corrosion essentially dictates the overall reaction process, acting as a "choke point" that controls how quickly vinyl acetate is produced.
The findings suggest fascinating implications for the future of catalyst design. Moving forward, researchers could explore how creating dual-function catalysts—capable of transitioning between solid and molecular forms—might optimize various chemical processes.
"Understanding the dual nature of these catalysts opens doors to countless applications, as we ponder whether other catalytic processes also harness this synergy," Harraz remarked.
The implications of these insights extend beyond vinyl acetate production; they signal a paradigm shift in how catalysis is perceived and studied. Educators like Christophe Coperet of ETH Zurich regard this groundbreaking work as a teaching opportunity, illustrating that the reconciliation between homogeneous and heterogeneous catalysis can simplify and clarify the complexities of chemical reaction mechanics.
This extraordinary research spells a future where catalysts are not limited to a single identity but can adapt, enhancing both efficiency and selectivity in chemical reactions. What other hidden interactions could be lurking in the world of chemistry? The future of catalysis is poised for revolutionary advancements!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)