Groundbreaking Discovery Reveals How Low Enzyme Levels Could Fuel Colorectal Cancer Malignancy
2025-04-01
Author: Wei Ling
Groundbreaking Discovery Reveals How Low Enzyme Levels Could Fuel Colorectal Cancer Malignancy
In a remarkable study conducted by cancer biologists in China, researchers have identified the critical role of a single enzyme in the conversion of healthy cells into invasive colorectal tumors—an intricate process characterized by various molecular mechanisms. Their findings underscore the need for further exploration of overlooked biological pathways contributing to one of the world's most prevalent cancers.
Colorectal Cancer Statistics
Colorectal cancer (CRC) ranks as the third most common cancer globally, with an alarming uptick in diagnoses among individuals under 50, particularly in the United States. A recent analysis by the American Cancer Society revealed that 20% of CRC cases in 2019 involved patients under 55—a rate that has doubled since 1995. Furthermore, there has been a 3% increase in advanced disease among this younger demographic, signaling an urgent need for specialized research and interventions.
Risk Factors
Traditionally associated with age, CRC risk factors include physical inactivity, obesity, low fiber intake, smoking, a family history of CRC or adenomas, inflammatory bowel disease, specific genetic disorders such as Lynch syndrome, and excessive alcohol consumption.
Research Details
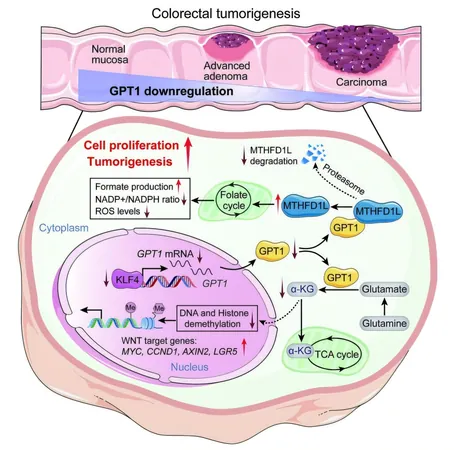
At the forefront of this critical research are scientists from the department of colorectal surgery at the Sixth Affiliated Hospital of Sun Yat-sen University in Guangdong. Collaborating with other institutions across China, they have discovered that a crucial enzymatic imbalance may be a driving factor in tumor formation. Specifically, the enzyme glutamic-pyruvic transaminase 1 (GPT1) has been shown to be significantly reduced in CRC patients, prompting researchers to investigate its association with tumor progression.
Findings of the Study
Lead researcher Li Xiong and his extensive team documented that lower levels of GPT1 correlate with poorer prognoses for CRC patients. Through experiments tracing the evolution of the disease—from normal cells to pre-cancerous conditions and finally to invasive cancer—the researchers delineated the importance of GPT1. The enzyme's levels declined sharply as the cancer progressed, establishing it as a vital participant in tumorigenesis.
Expert Insights
'Colorectal cancer often follows the sequence of normal tissue to adenoma to carcinoma (N-A-C),' Xiong states. Despite many molecular mechanisms still remaining unknown in colorectal adenoma carcinogenesis, this study highlights the causative role low GPT1 plays in the disease's progression.
Emerging Treatment Approaches
Crucially, the research team also identified that poliumoside, a compound that activates GPT1, can suppress tumor growth—a promising direction for future therapies. With advanced stages of CRC showing persistently poor survival rates, the emergence of such potential treatments highlights the need for innovative therapeutic strategies.
Importance of Early Detection
The research emphasizes the criticality of early detection and removal of precancerous lesions, as approximately 85% of CRC cases develop from adenomas. However, patients who have undergone adenoma removal may still face an increased risk of developing new adenomas or CRC. To better manage these risks, the US Multi-Society Task Force on CRC and the European Society of Gastrointestinal Endoscopy recommend colonoscopy surveillance within three years following the removal of advanced adenomas larger than 10 millimeters.
Towards Better Risk Assessment
Despite these recommendations, Xiong argues that effective indicators for assessing the risk of adenoma transformation are still lacking, complicating the ability to provide timely interventions for high-risk patients and avoiding unnecessary procedures for low-risk individuals.
Laboratory Findings
In their laboratory studies, Xiong's team analyzed normal colorectal tissues, adenomas, and cancerous tissues collected from CRC patients, finding a significant deficiency in GPT1 levels—which corresponded to poorer clinical outcomes. Their work further established that GPT1 plays a crucial role in tumor suppression by producing α-ketoglutarate, a metabolic molecule that inhibits the WNT signaling pathway, involved in critical cancer-related processes.
Poliumoside's Potential
The investigation into poliumoside's potential revealed it could reactivate GPT1, significantly slowing tumor growth in both patient-derived organoids and mouse models. These findings suggest a promising avenue for clinical trials, aiming to develop innovative therapies based on metabolic reprogramming.
Conclusion
'In this study, we pinpointed GPT1 as a key regulator in the metabolic reprogramming and progression of colorectal cancer,' Xiong concluded. 'The deficiency of GPT1 stimulates CRC tumorigenesis by altering cellular metabolism in both enzyme-dependent and independent manners.'
Future Prospects
Stay tuned as more updates unfold from this pivotal research, promising new hope in the fight against colorectal cancer!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)