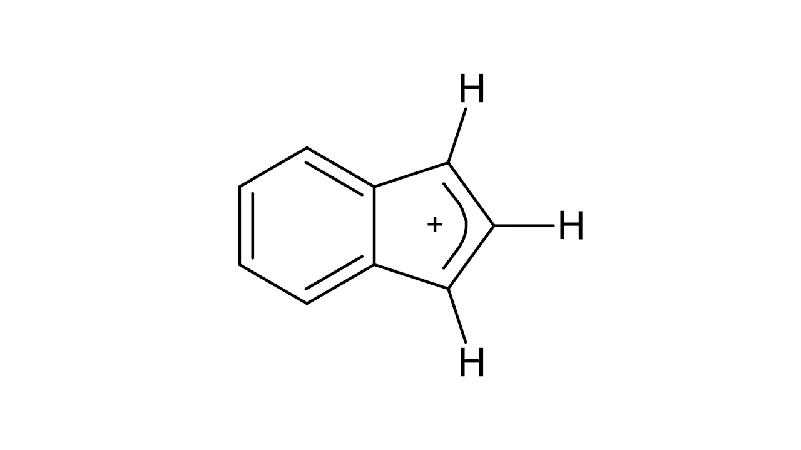
Groundbreaking Discovery: How the Indenyl Cation Survives in Space!
2025-03-31
Author: John Tan
Groundbreaking Discovery: How the Indenyl Cation Survives in Space!
In an astonishing revelation from the Taurus Molecular Cloud-1, researchers have identified several small polycyclic aromatic hydrocarbons (PAHs) that boast a closed-shell electronic structure. This discovery could reshape our understanding of chemical stability in the universe.
A recent study observed the indenyl cation (C9H+7) exhibiting remarkable radiative cooling properties via a combination of recurrent fluorescence (RF) and infrared (IR) emission. The findings indicate that this cation, when generated with vibrational energy levels up to Ec = 5.85 eV, remains stable instead of breaking apart, which is nearly 2 eV above its dissociation threshold.
This behavior is not an isolated phenomenon. The efficient radiative stabilization observed in the indenyl cation is expected to be characteristic of other closed-shell PAHs in space, possibly explaining their prevalence throughout the cosmos. This discovery not only enhances our understanding of molecular chemistry in cold environments but also opens pathways for future research in astrobiology and the formation of complex organic molecules in space.
With such remarkable findings, scientists are now eager to explore the implications of these stable structures for the origins of life and chemical evolution in space. Stay tuned as we dive deeper into the cosmos and unravel the secrets held by these molecular wonders!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)