Groundbreaking Discovery: How Electric Double Layers Form at Battery Nucleation Sites!
2025-08-06
Author: Nur
Unlocking the Mysteries of Battery Interactions
Batteries aren’t just power sources; they are intricate technological marvels that intertwine chemistry, physics, materials science, and electronics. Beyond their everyday applications ranging from smartphones to electric vehicles, researchers are diving deep into the hidden intricacies of these devices at the molecular level.
A Revolutionary Study by Leading Researchers
A pioneering team, spearheaded by Yingjie Zhang, a distinguished professor of materials science at the University of Illinois Urbana-Champaign, has conducted an exploration into a crucial yet often overlooked element of electrochemical cells: the liquid interfaces at solid boundaries. Their findings, recently published in *Proceedings of the National Academy of Sciences*, shine a light on the elusive structures known as electrical double layers (EDLs) that form in response to chemical changes at these critical interfaces.
The Fascinating Role of Electrical Double Layers
Traditionally, these EDLs have been acknowledged primarily as mediators of voltage differences between liquid electrolytes and solid conductors. They consist of self-organized electrolyte layers just nanometers thick, maintaining the delicate balance of mobile charges essential for battery function.
Breaking New Ground in Understanding Interfaces
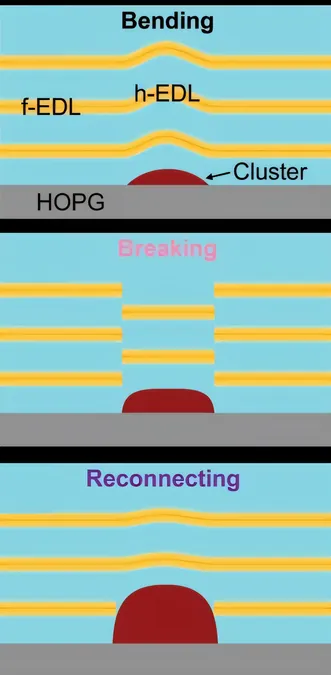
Despite decades of research, many studies have concentrated on simplified systems featuring flat, uniform surfaces, leaving a significant gap in our understanding of the variability that occurs in actual electrochemical cells. This investigation aimed to unravel those complexities by utilizing advanced 3D atomic force microscopy to examine the molecular architecture of non-uniform EDLs surrounding surface clusters, a pioneering effort.
Key Insights from Advanced Microscopy Techniques
The team uncovered three primary behaviors of EDLs in the presence of these surface clusters: 1. **Bending**: EDLs curve around the surface clusters. 2. **Breaking**: Portions of the EDL detach, forming new intermediate layers. 3. **Reconnecting**: EDLs merge with nearby layers, even with offsets in their structure. According to lead author Qian Ai, these patterns are not just specific to their current study but could apply universally to other systems based on the surface morphology of solids.
A New Chapter in Electrochemistry
Zhang emphasized the monumental implications of their findings: "Recognizing EDLs in real, heterogeneous systems is a monumental achievement in electrochemistry. Not only do these revelations hold practical potential for technological advancements, but they also pave the way for rethinking electrochemistry textbooks moving forward."
What Lies Ahead for Electrochemical Research?
As this groundbreaking research unfolds, the team is eager to explore further applications of their work. The continued investigation into these molecular phenomena could revolutionize battery technology and elevate our understanding of electrochemical cells into a new era!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)