Groundbreaking Discovery Could Revolutionize Treatment for Congenital Spinal Defects!
2025-03-17
Author: Wei Ling
Introduction
In a significant breakthrough, researchers at Northwestern Medicine have unveiled previously unknown mechanisms essential for spinal column development during embryonic stages. This study, recently published in *Nature Communications*, holds promise for developing new treatments for congenital scoliosis and related birth defects.
Understanding the Vertebral Column
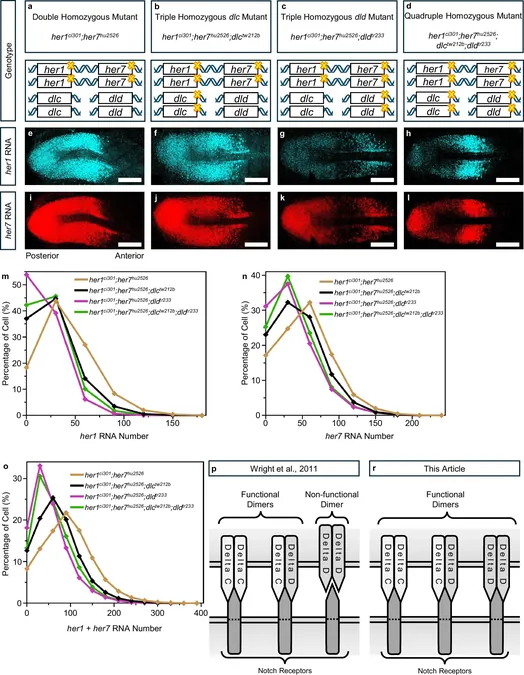
The vertebral column in vertebrate species, including humans, is segmented into vertebral discs, providing both flexibility and support. These discs form from specialized cells known as somites during early embryonic development, which are sequentially divided in a process orchestrated by a biological mechanism referred to as the vertebrate segmentation clock.
The Role of the Segmentation Clock
Ertugrul Özbudak, Ph.D., the senior author of the study and a professor of Cell Biology at Northwestern, explained that this segmentation clock involves the oscillating expression of certain genes that code for vital proteins. 'It’s like a rhythmic dance where genes express RNA and proteins in a wave-like manner,' he elaborated.
Key Proteins in Somite Development
Each somite cell contains two critical proteins, Her1 and Her7, instrumental in regulating this biological clock. Simultaneously, proteins DeltaC and DeltaD engage with the Notch signaling pathway, stimulating the transcription of segmentation clock genes in adjacent presomitic cells. Mutations in these genes can lead to serious spinal segmentation defects in humans, including congenital scoliosis—a condition where the spine curves abnormally.
Collaboration of Cellular Mechanisms
Özbudak emphasized the collaborative nature of this process: 'Hundreds of cells must synchronously produce and degrade these signaling molecules, which necessitates effective cell-to-cell communication.'
New Discoveries: DeltaD's Role
Until now, it was believed that only DeltaC played a role in transmitting signals leading to gene transcription. However, through advanced imaging techniques applied to various genetic types of zebrafish embryos, Özbudak's team discovered that DeltaD is not only functional but also enhances the transcription levels of both DeltaC and DeltaD. 'DeltaC is critical for immediate synchronization, while DeltaD boosts the levels needed for effective oscillation and coordination,' he noted.
Implications for Future Research
These revelations are expected to deepen our comprehension of how mutations in these genetic pathways cause congenital spine abnormalities and will pave the way for innovative therapeutic strategies. Building upon this research, Özbudak's team is poised to investigate the roles of other transcription regulators and cell signaling mechanisms in spinal development.
Conclusion
As scientists continue to delve into the mysteries of genetic regulation, this research stands on the brink of delivering new hope for those affected by congenital spinal defects. Stay tuned for updates on potential treatments that could change lives!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)