Groundbreaking Discovery: A New Biomarker Unveils Secrets of Aging Cells!
2024-12-25
Author: Sarah
Introduction
In an exciting breakthrough, researchers at the Mayo Clinic have identified interleukin-23 receptor (IL-23R) as a crucial biomarker for cellular senescence and aging, applicable to both mice and humans. This discovery sheds light on the aging process and opens the door to novel therapeutic interventions aimed at combating age-related diseases.
Understanding Cellular Senescence
As we age, our cells undergo a transformation known as cellular senescence, where they cease to divide and enter a state often likened to a “zombie” phase. In this state, these cells remain metabolically active but contribute to inflammation instead of self-destruction, leading to impaired cell signaling and an increase in pro-inflammatory cytokines. This phenomenon has been linked to a multitude of age-related ailments, affecting systems including the immune, cardiovascular, and neurological profiles.
Historical Context and Biomarker Search
Historically, scientists have searched for reliable biomarkers to gauge the levels of active senescent cells, which could revolutionize early disease detection and facilitate preemptive clinical interventions. The recent study, titled "IL-23R is a senescence-linked circulating and tissue biomarker of aging," published in *Nature Aging*, meticulously investigates senescence-related biomarkers by measuring their responsiveness to various therapeutics in different age groups of mice.
Research Methodology and Findings
During the research, scientists evaluated 92 plasma proteins utilizing the Olink Target 96 Mouse Exploratory panel, eventually narrowing it down to 67 suitable candidates. A thorough examination involving multiple tissue types—such as the kidney, liver, spleen, cerebral cortex, adipose tissue, and lung—was performed using real-time PCR to assess 21 gene expressions related to senescence and inflammation.
Senolytic Drugs and Their Impact
Furthermore, the researchers employed senolytic drugs, including venetoclax, navitoclax, fisetin, and luteolin, alongside transgenic approaches, to specifically target p16-positive senescent cells. Following these interventions, remarkable changes were observed in both plasma proteins and tissue transcripts.
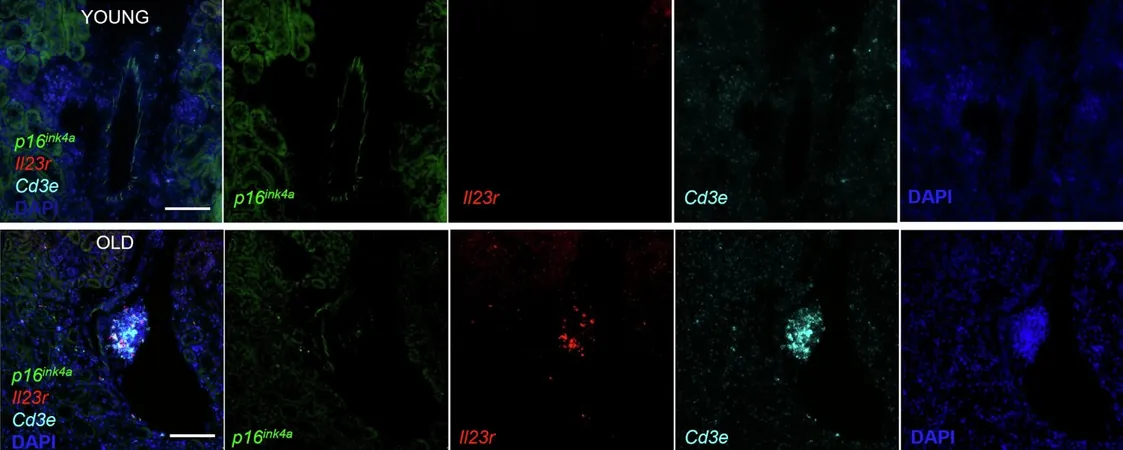
IL-23R as a Key Biomarker
Among the tested proteins, IL-23R, along with CCL5 and CA13, evidenced age-related shifts in both circulation and tissue expression. Notably, senolytic treatments reversed the age-dependent increases of IL-23R and CCL5, while CA13 levels, typically diminished with aging, were restored to youthful benchmarks.
Conclusion and Future Implications
What sets IL-23R apart as the standout biomarker is its strong and consistent association with the aging process across various tissues. Its levels rise significantly with age in both mice and humans, reflecting a robust response to senolytic therapies. This paves the way for IL-23R to serve as a reliable indicator of systemic senescent cell burden.
The implications of this research are monumental. By establishing IL-23R as a potential biomarker for aging, researchers have set the stage for groundbreaking advancements in diagnosing and treating age-related diseases, possibly enabling early detection and interventions before clinical symptoms emerge.
With this newfound knowledge, the fight against aging and its associated maladies has garnered a powerful ally. Could this biomarker be the key to unlocking a longer, healthier life? Only time will tell, but the prospects are undoubtedly promising!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)