Groundbreaking Discoveries Illuminate New Pathways for Treating Estrogen Receptor-Negative Breast Cancer
2025-04-07
Author: Rajesh
Introduction
In an exciting new study published in Science Advances, researchers from Northwestern Medicine have unveiled previously unrecognized metabolic alterations that could play a crucial role in the onset of estrogen receptor–negative (ERneg) breast cancer. This significant research, spearheaded by Susan Clare, M.D., Ph.D., and Seema Khan, M.D., promises to inspire innovative therapeutic strategies for patients grappling with limited treatment options.
Background on ER-Negative Breast Cancer
Approximately 15% of breast cancer cases are classified as hormone-receptor negative, meaning the cancer cells lack the receptors that typically bind estrogen or progesterone, crucial hormones for tumor growth. This classification presents a relentless challenge, as conventional hormone therapies often yield little to no benefit for these patients.
Previous Investigations
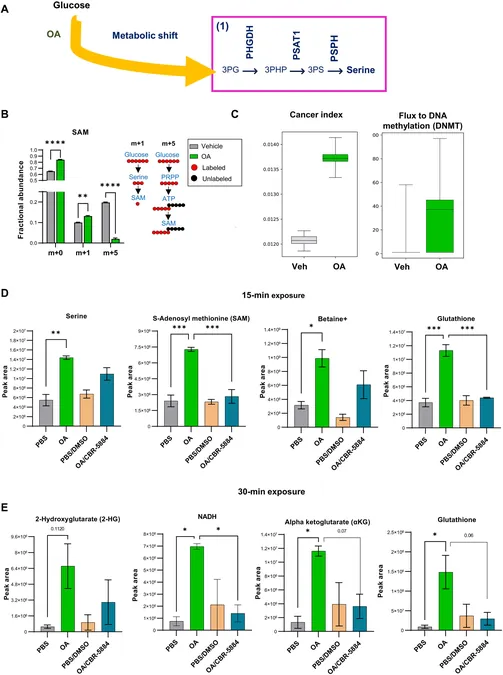
Previous investigations by Dr. Khan highlighted that in patients diagnosed with ERneg breast cancer, healthy epithelial cells from the breast opposite the tumor exhibited elevated levels of a specific lipid metabolism gene signature. This shift towards lipid metabolism has been linked to the development of ERneg breast cancer through two critical pathways: serine, one-carbon, glycine (SOG), and methionine pathways. These pathways are known to influence protein methylation, which plays a vital role in gene regulation.
Current Study Insights
In the current study, the research team utilized advanced techniques like metabolomics, epigenomic profiling, and single-cell RNA sequencing to analyze human breast epithelial cell lines along with healthy breast tissue that had been exposed to medium-chain fatty acids. The results were compelling.
"Our findings reveal that exposure to fat triggers significant changes in metabolism, histone methylation, and gene expression — all of which correlate with hormone-receptor negative breast cancer," Dr. Khan emphasized. This research underlines the connection between lipid metabolism and ERneg breast cancer, particularly focusing on an enzyme known as phosphoglycerate dehydrogenase (PHGDH). When medium-chain fatty acids are present, PHGDH encourages a metabolic shift towards the SOG and methionine pathways.
Role of PHGDH
Remarkably, the study found that PHGDH is pivotal in increasing the production of S-adenosylmethionine, a compound that regulates gene expression through methylation, alongside an oncometabolite called 2-hydroxyglutarate. This metabolite leads to epigenomic reprogramming in breast epithelial cells, heightening interest in its potential as a target for therapy.
Antioxidant Activity and Implications
Furthermore, this metabolic transition is associated with an increase in the antioxidant glutathione, which plays a protective role by mitigating oxidative stress and enhancing cell survival following DNA damage.
Conclusion and Future Directions
The research aligns with several established hallmarks of cancer, such as aberrant cellular metabolism and evasion of programmed cell death, thereby suggesting new avenues for preventive measures and treatments. Dr. Khan expressed her hope that these promising findings will facilitate the identification of risk factors for women specifically susceptible to ER-negative breast cancer, ultimately paving the way for novel preventive strategies.
This groundbreaking study marks a leap forward in understanding the complex metabolism of breast cancer cells and sets the stage for targeted interventions that could significantly improve outcomes for patients battling this challenging cancer subtype. Stay tuned for further developments as this research progresses, which could transform the landscape of breast cancer treatment!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)