Groundbreaking Astrocyte Study Uncovers New Hope in the Fight Against Alzheimer's Disease!
2024-09-23
Alzheimer's disease continues to be a significant global health challenge, with approximately 50 million individuals affected by various forms of dementia. Among these, Alzheimer's is the most prevalent, characterized by progressive memory loss and cognitive decline, severely disrupting daily life. Alarmingly, there is still no cure available for this devastating condition.
The most common variant is sporadic Alzheimer's disease, which arises from a complicated interplay of genetic, environmental, and lifestyle factors without a clear familial connection. However, a newly published study from the University of Sheffield has opened exciting possibilities for therapeutic intervention by focusing on a type of brain cell called astrocytes.
Astrocytes are crucial components of the nervous system, outnumbering neurons and fulfilling essential roles such as nutrient supply, structural support, and insulation for neurons. Yet, early stage Alzheimer’s patients demonstrate a malfunction in astrocytic metabolism, hampering the energy production vital for optimal neuron function. "Many researchers concentrate solely on neurons, but astrocytes vastly outnumber them. When their metabolism falters, so does the support they offer to neurons," explains Professor Heather Mortiboys from the Neuroscience Institute, emphasizing the importance of this research.
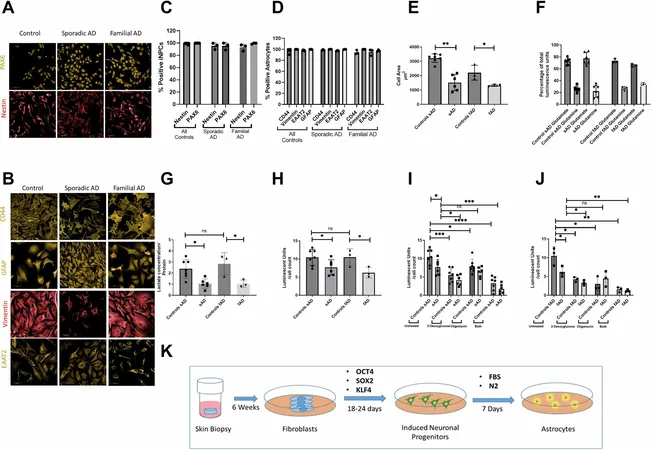
Dr. Simon Bell’s groundbreaking study provides new insights into the role of astrocytes in sporadic Alzheimer's disease, focusing on the mitochondria—often referred to as the cell’s energy powerhouses. The findings indicate that astrocytes afflicted by Alzheimer's disease produce less energy and experience increased mitochondrial stress, impairing overall brain function.
Published in the respected journal *Molecular Psychiatry*, the study outlines that a key enzyme, hexokinase 1 (HK1), is significantly reduced in astrocytes from patients with sporadic Alzheimer's. The research demonstrates that enhancing HK1 levels could substantially boost the energy capabilities of these astrocytes, potentially countering the detrimental effects of their dysfunction.
With a team of innovative researchers including Hollie Wareing and Professor Dame Pamela J. Shaw, Dr. Bell utilized skin biopsies from Alzheimer's patients to cultivate fibroblast cells in the lab. These were reprogrammed into stem cells and subsequently differentiated into astrocytes, enabling a direct comparison between astrocytes from sporadic and familial Alzheimer’s cases.
Professor Mortiboys noted profound differences in the astrocytic mitochondria, with variations in shape, size, and altered glycolytic pathways, leading to an overall energy deficiency. "This study not only reveals the distinct mechanisms underlying sporadic versus familial Alzheimer's but also highlights how elevating HK1 could restore metabolic function in the early stages of the disease," she remarked.
This pioneering research signifies a crucial step in understanding Alzheimer’s disease at a cellular level and presents a promising avenue for therapeutic target development centered around astrocyte metabolism. As researchers continue to unravel the complexities of Alzheimer's, there is growing hope that new treatments could soon emerge, turning the tide in the battle against this heartbreaking condition. Stay tuned for more groundbreaking developments that could change the future of Alzheimer's treatment!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)